Interesting Science Videos
What is Streptococcus agalactiae?
Streptococcus agalactiae is a Gram-positive, non-motile, non-spore-forming coccus that is the only member of the Group B of the Lancefield antigen grouping.
- It is a β-hemolytic, catalase-negative, facultative anaerobe that consists of ten different serotypes, separated on the basis of the immunologic reactions of their capsular polysaccharide.
- Because it is the only species present in the Lancefield Group B, S. agalactiae is also called the Group B Streptococcus (GBS).
- S. agalactiae is a part of the human normal flora and colonizes areas like the gastrointestinal tract and the genitourinary tract of most adults, but it is also associated with severe invasive infections, mostly in neonates, pregnant women, older adults, and other immunocompromised individuals.
- It is considered a pathobiont that converts from the asymptomatic mucosal carriage state to a major bacterial pathogen causing severe invasive infections. It is now a leading cause of neonatal meningitis and septicemia.
- It is also found in cattle where it is considered a veterinary pathogen as it causes bovis mastitis in dairy cows.
- In addition to humans and cattle, S. agalactiae has been isolated from animals such as chickens, dogs, dolphins, horses, lizards, camels, cats, fish, frogs, mice, and monkeys.
- The species name ‘agalactiae‘ is taken from the Greek term ‘agalactia’, meaning ‘want of milk’ as it causes inflammation of the udder.
- It was first differentiated from other streptococci by Rebecca Lancefield in the 1930s after it was isolated from the milk of cows with bovine mastitis.
Classification of Streptococcus agalactiae
The genus Streptococcus is differentiated from other lactic acid bacteria that produce lactic acid as a sole or major endproduct of carbohydrate metabolism. The genus consists of more than 50 different species, most of which are categorized into ‘species group’ based on one or more similar characteristics. Based on 16S rRNA gene sequence analysis the genus Streptococcus belongs within the low (< 50 mol%) G+C branch of the Gram-positive eubacteria, and is a member (type genus) of the family Streptococcaceae. S. agalactiae is one of the thirteen species that belong to the pyogenic groups of streptococci, most of which are characterized by b-hemolytic activity and its 16S rRNA sequencing. The species found in humans are also termed hemolytic streptococci. It is also the only species present in the Lancefield Group B based on the presence of B antigen on its capsular polysaccharide.
The following is the taxonomical classification of S. agalactiae:
| Domain | Bacteria |
| Phylum | Firmicutes |
| Class | Bacilli |
| Order | Bacillales |
| Family | Streptoococcaceae |
| Genus | Streptococcus |
| Species | S. agalactiae |
Habitat of Streptococcus agalactiae
- Both humans and cattle are the two major permanent hosts of S. agalactiae. It was first isolated from cattle by Lehmann and Neumann in 1896 but was later found to be the part of the human normal flora colonizing different areas of the human body.
- In humans, it primarily colonizes the gastrointestinal tract and the genitourinary tract, whereas, in cattle, it is mostly found in the udder.
- The gastrointestinal tract is recognized as a major reservoir for S. agalactiae and possibly is the source of vaginal colonization.
- Besides humans and cattle, it has also been isolated different other animals like dogs, cats, dolphins, monkeys, crocodiles, mice, etc.
- It is considered a pathobiont as it can convert from the asymptomatic mucosal carrier state to a major bacterial pathogen, resulting in severe invasive infections.
- Before it was isolated from humans, it was only considered a veterinary pathogen as it caused bovis mastitis or inflammation of the udder in dairy cows.
- It is transferred to the newborns from the infected or asymptomatic mothers via the birth canals during birth.
- The presence of the capsular polysaccharide around the bacteria allows the organism to survive in the gastrointestinal tract and protect itself against the immune invasion.
Morphology of Streptococcus agalactiae
- The cells of S. agalactiae are spherical or ovoid Gram-positive cocci of the size 0.6–1.2 µm in diameter. However, some species may develop rod-like cells depending on the growth conditions.
- The arrangement of the cells is characteristic of all Streptococci as the cells are arranged in chains, occurring in chains of seldom less than four cells and frequently in pairs or very long chains. The chains might be longer if the bacteria originate from a fluid culture.
- Cross-walls during cell division are formed at right angles to the chain, and after division, an appearance of pairing may remain.
- The organism is surrounded by a bacterial capsule composed of polysaccharide or exopolysaccharide that surrounds and protects the bacterium by preventing complement deposition and opsonization.
- The capsular polysaccharide is covalently bound to the cell wall peptidoglycan, thus creating the mucoid capsule layer covering the bacterial surface.
- The cell wall of S. agalactiae is composed of the typical peptidoglycan, along with various carbohydrate structures like teichoic acids, and a number of proteins.
- The peptidoglycan present on the cell wall of S. agalactiae is of the type Lys–Ala1–3(Ser) and the group B-specific polysaccharide antigen is composed of rhamnose, N-acetylglucosamine, and galactose. This antigen not thought to be a virulence factor of the organism, nor is it important in natural immunity; however, it is a useful tool to identify these organisms in the clinical laboratory.
- The cell membrane had a lipid-protein bilayer that helps in the transport of different molecules in and out of the cells through different transport channels or systems.
Cultural characteristics of Streptococcus agalactiae
The growth of Streptococcus on ordinary nutrient media is generally low in contrast to that of other Gram-positive species. Growth is more profuse on media enriched with blood, serum, or a fermentable carbohydrate. To avoid competition and to inhibit other Gram-positive organisms, Selective Strep Agar is used as a selective media. Many strains are capable of growing in media containing 40% bile. Some strains produce a yellow, orange, or brick-red pigment, and the growth may be enhanced by the addition of starch to the medium or by incubation with 5% carbon dioxide. Ideal growth is observed at the temperature of 20-35°C, whereas no growth is observed at either 10°C or 45°C. Growth is observed under neutral to acidic pH, but no growth is seen beyond the pH of 9.
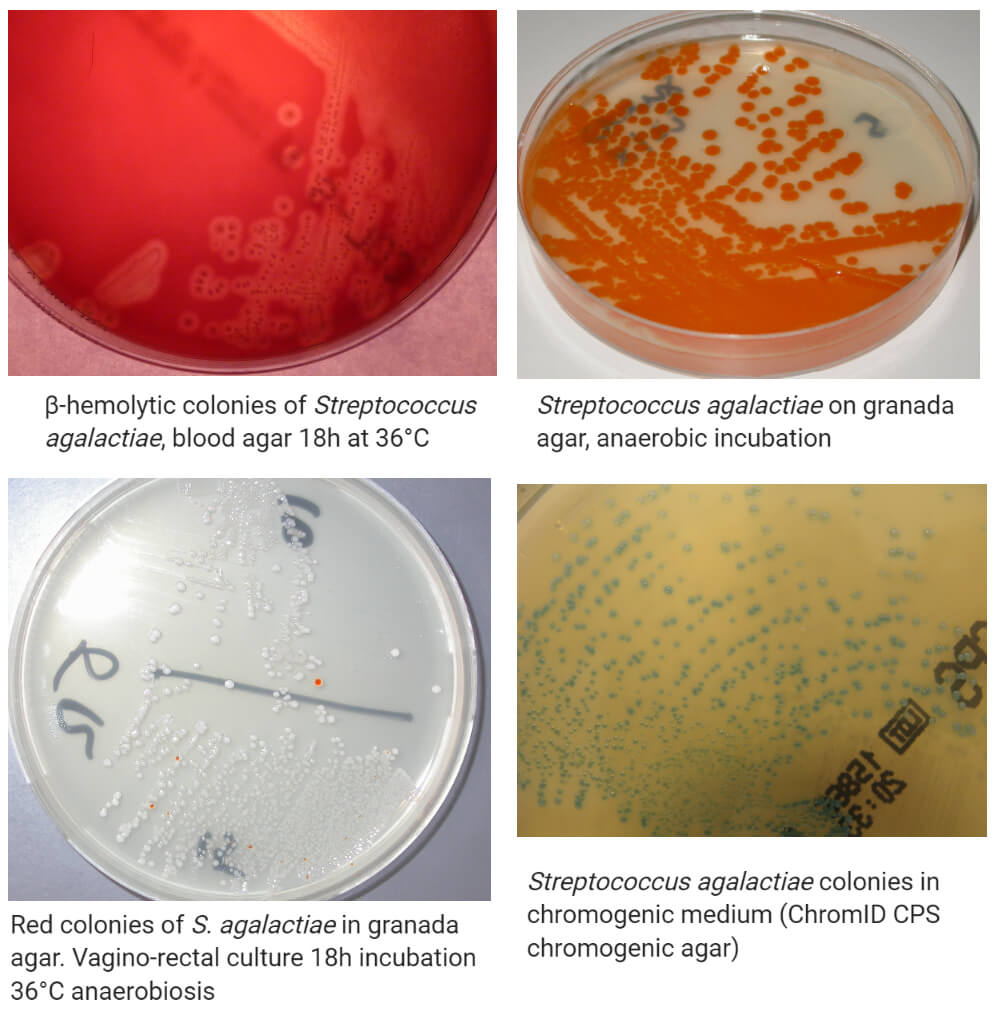
Image Source: Wikipedia.
The following are some cultural characteristics of S. agalactiae on different culture media:
1. Nutrient Agar (NA)
- White to grey colored colonies of an average size of 1 mm in diameter. The colonies were round with raised elevation and an entire margin.
- Growth is mostly poor and requires air with supplied carbon dioxide.
2. Blood Agar (BA)
- Typical smooth, non-pigmented, convex colonies with entire margin are observed on blood agar.
- Growth occurs readily on blood agar and exhibits various types of hemolysis viz. typical β-hemolysis, but with a narrow zone, α-double zone, or no hemolysis.
- The CAMP factor produced by most group B streptococci binds to erythrocyte membrane altered by Staphylococcus aureus sphingomyelinase C. This result in the unique ‘arrowhead’ pattern of hemolysis is seen on sheep blood agar when GBS is grown near colonies of S. aureus.
Biochemical characteristics of Streptococcus agalactiae
The biochemical characteristics of S. agalactiae can be tabulated as follows:
| S.N | Biochemical Characteristics | S. agalactiae |
| 1. | Capsule | Capsulated |
| 2. | Shape | Cocci |
| 3. | Catalase | Negative (-) |
| 4. | Oxidase | Negative (-) |
| 5. | Citrate | Negative (-) |
| 6. | Methyl Red (MR) | Negative (-) |
| 7. | Voges Proskauer (VR) | Positive (+) |
| 8. | OF (Oxidative-Fermentative) | Facultative anaerobes |
| 9. | Coagulase | Negative (-) |
| 10. | DNase | Negative (-) |
| 11. | Clumping factor | Negative (-) |
| 12. | Gas | Negative (-) |
| 11. | H2O2 | Negative (-) |
| 12. | Hemolysis | α, β, non-hemolytic |
| 13. | Motility | Non-motile |
| 14. | Nitrate Reduction | Negative (-) |
| 15. | Gelatin Hydrolysis | Negative (-) |
| 16. | Pigment Production | Variable |
| 17. | Bile esculin test | Negative (-) |
| 18. | CAMP reaction | Positive (+) |
| 19. | PYR test | Negative (-) |
| 20. | Bacitracin resistance | Resistant |
| 21. | Lancefield group | Group B |
Fermentation
| S.N | Substrate | S. agalactiae |
| 1. | Glucose | Positive (+) |
| 2. | Fructose | Positive (+) |
| 3. | Galactose | Positive (+) |
| 4. | Lactose | Positive (+) |
| 5. | Maltose | Positive (+) |
| 6. | Mannitol | Negative (-) |
| 7. | Mannose | Negative (-) |
| 8. | Raffinose | Negative (-) |
| 9. | Ribose | Positive (+) |
| 10. | Sucrose | Positive (+) Extracellular polysaccharide (dextran) is produced from sucrose. |
| 11. | Starch | Positive (+) |
| 12. | Trehalose | Positive (+) |
| 13. | Xylose | Negative (-) |
| 14. | Salicin | Positive (+) |
| 15. | Glycerol | Positive (+) |
| 16. | Dulcitol | Negative (-) |
| 17. | Cellobiose | Positive (+) |
| 18. | Rhamnose | Negative (-) |
| 19. | Arabinose | Negative (-) |
| 20. | Inulin | Negative (-) |
| 21. | Sorbitol | Positive (+) |
| 22. | Pyruvate | Negative (-) |
| 23. | Glycogen | Negative (-) |
Enzymatic Reactions
| S.N | Enzymes | S. agalactiae |
| 1. | Acetoin | Positive (+) |
| 2. | Acid Phosphatase | Not determined |
| 3. | Alkaline Phosphatase | Positive (+) |
| 4. | Ornithine Decarboxylase | Not determined |
| 5. | Hyaluronidase | Positive (+) |
| 6. | β-D-galactosidase | Negative (-) |
| 7. | Arginine Dehydrolase | Negative (-) |
| 8. | Neuraminidase | Positive (+) |
| 9. | Urease | Negative (-) |
- S. agalactiae can hydrolyze arginine but cannot hydrolyze esculin and gelatin.
- They can tolerate 6.5% NaCl but cannot tolerate higher concentrations than that.
Virulence Factors of Streptococcus agalactiae
As in all infections, S. agalactiae infection also has to deal with a number of diverse cell types such as macrophages, epithelial cells, and endothelial cells during the invasive disease process. To overcome these defensive barriers and survive in the host, an organism must possess a variety of virulence factors. Such virulence factors not only allow invasion of the host tissue, resulting in mild to severe diseases but also protect the organism against the immune response of the host’s body. S. agalactiae also has several such mechanisms or factors that protect and allow the organism to cause different types of infections.
Some of such virulence factors found in S. agalactiae are given below:
1. Capsule
- The capsular polysaccharide serves as the basis for serotyping of GBS in the reference laboratory and is also considered a major virulence factor of GBS.
- The capsular polysaccharide is made up of more than 100 repeating units of the monosaccharides galactose, glucose, N-acetylglucosamine, and N-acetylneuraminic acid (sialic acid).
- The primary function of the GBS capsule is thought to be the protection of the organism from phagocytosis by the host’s immune system.
- The sialic acid component of the capsule inhibits the alternative pathway of complement by preventing the deposition of active C3 complement on the surface of GBS.
- If C3 does deposit, the capsule promotes the conversion of C3b to iC3b on the bacterial surface, resulting in the organism being resistant to uptake and killing by neutrophils.
2. Lipoteichoic acid
- Lipoteichoic acids (LTA) are cell wall polymers containing glycerol phosphate or ribitol phosphate that is found in most Gram-positive bacteria.
- Various functions have been associated with this polymer, one of which is mediating adherence of Grampositive bacteria to eukaryotic cells.
- These have also been demonstrated to bind to the cell membranes of erythrocytes and epithelial cells.
- It is found that LTA binding to human fetal and embryonic epithelial cells occurs in a two-step process; the initial step being hydrophobic interactions between host cells and GBS, followed by the interaction of the glycerolphosphate backbone with the embryonic eukaryotic cell surface.
- As a result, lipoteichoic acid facilitates the binding of the bacteria to the cell surface of epithelial cells in both adults and neonates.
3. Beta hemolysin
- Group B streptococcus characteristically shows a narrow zone of beta hemolysis on sheep blood agar, and this is used as one of the first phenotypic features in identifying this organism in the clinical laboratory.
- The beta-hemolysin is a pore-forming, non-immunogenic cytolysin that is active against a variety of cell types.
- The beta-hemolysin produced by S. agalactiae has been shown to promote the induction of interleukin-8 (IL-8), a potent neutrophil chemotactic agent.
- In addition to being a host signal to initiate innate immune responses to the organism, IL-8 mediated neutrophil recruitment may also contribute to destructive acute inflammatory processes seen in some cases of invasive Group B streptococcus disease.
- The injured cells show membrane disruptions, cellular swelling, changes in organelles, and chromatin and lactate dehydrogenase release.
4. Hyaluronate lyase
- Hyaluronate lyase is a protein virulence factor of S. agalactiae that can enzymatically degrade hyaluronic acid, a predominant component of the extracellular matrix of animal connective tissue and nervous system.
- The function of hyaluronate lyase is to act as a spreading factor, destroying the normal connective tissue structure of the host and promoting bacterial dissemination.
- Additionally, both amniotic fluid and placenta contain a high concentration of hyaluronic acid so the enzyme may aid the organism in traversing this barrier to gain access to the fetus.
Pathogenesis of Streptococcus agalactiae
Streptococcus agalactiae is an important human pathogen that causes different severe neonatal as well as adult infections. The course of infection by S. agalactiae begins with the colonization and invasion of a number of different host compartments. Different virulence factors present in the bacteria like the polysaccharide capsule, the hemolysin, the C-proteins, the hyaluronate lyase, and the lipoteichoic acid, and a number of unknown bacterial components are involved in the pathogenesis of infection.
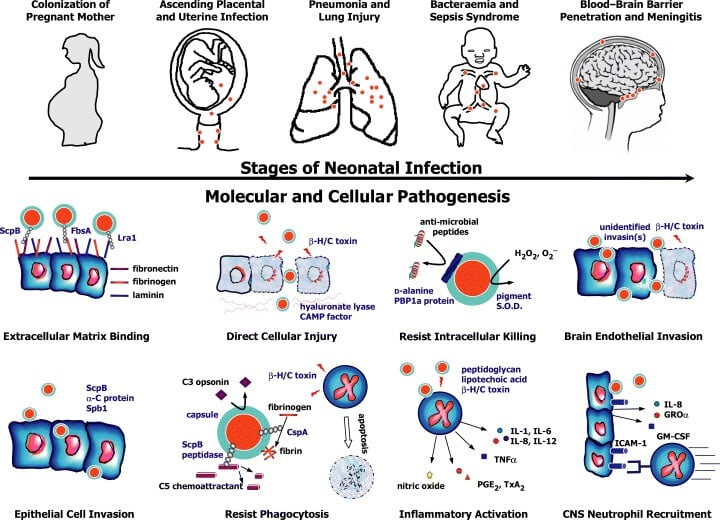
Figure: Stages in the molecular and cellular pathogenesis of neonatal group B Streptococcal (GBS) infection. β‐H/C, beta-hemolysin/cytolysin; S.O.D., superoxide dismutase; IL, interleukin; TNFα, tumor necrosis factor‐alpha; PGE2, prostaglandin E2; TxA2, thromboxane A2; GROα, growth‐related oncogene‐alpha; ICAM‐1, intercellular adhesion molecule 1; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor. Image Source: https://doi.org/10.1111/j.1365-2958.2004.04266.x
The following is the pathogenesis of infections caused by S. agalactiae:
1. Transmission
- Maternal colonization and vertical transmission of S. agalactiae are found in more than 95% of neonatal carriers, except for rare cases of transmission through nursery personnel or human milk.
- The colonization of the birth canal and perinatal transmission of S. agalactiae play a vital role in the pathogenesis of GBS disease.
- Most of the infections develop in the uterus, and the ascending bacteria reach the amniotic fluid, and the intake of contaminated fluid by the infant leads to the development of the invasive disease.
- However, penetration of S. agalactiae through intact membranes can also occur, leading to severe cases of intrauterine infection or abortion.
2. Colonization
- Colonization of the maternal genitourinary or gastrointestinal tract is the most important risk factor for GBS disease.
- Different factors like the proteinaceous component LTA of the bacterial cell wall and the surface proteins are assumed to be necessary for the adhesion of S. agalactiae to vaginal epithelial cells, buccal epithelial cells, pulmonary epithelial cells, and endothelial cells.
- Studies have demonstrated that streptococci adhere in two steps; the first is a relatively weak and reversible first interaction mediated by components of the cell wall and a second interaction mediated by proteins, leading to a firm adhesion of bacteria to eukaryotic cells.
- The adhesion of S. agalactiae to extracellular matrix proteins has been described for fibronectin and fibrinogen, but the corresponding adhesins on the streptococcal surface have not been defined.
- However, a novel surface protein (Lmb) of S. agalactiae has been identified that mediates the binding to human placental laminin, the major component of the placental basement membrane.
- Heavy colonization with S. agalactiae is with premature rupture of membranes, and several cases of penetration of S. agalactiae through intact membranes have been seen.
- In these situations, the bacteria get into close contact with basement membranes and Lmb protein might contribute to the ability of S. agalactiae to overcome the mucosal barrier.
3. Invasion
- During infection, S. agalactiae encounters a number of different barriers.
- In some cases of GBS disease, infection of the infant occurs through intact chorioamniotic membranes, requiring the bacteria to transverse chorion cells, amnion cells, and the placental basement membrane.
- S. agalactiae can enter different eukaryotic cells and has the ability to survive inside these cells, which is an essential mechanism for the invasion of different host compartments.
- Bacterial cells are taken up by active endocytosis, and inside the cells, bacteria are found within vacuoles.
- The ability of the organism to invade eukaryotic cells in vitro results in the ability to cause invasive infection.
- The invasion of eukaryotic cells is mediated through proteins of the cell surface and the capsular polysaccharide.
- Following evasion, the bacteria might enter the lower respiratory tract where numerous bacteria enclosed in atypical hyaline membranes after evading the host immune system.
- β-hemolysin produced by the bacteria results in marked pulmonary epithelial and endothelial cell injury, ultimately leading to pneumonia.
- Further intravascular invasion of bacteria and failure of the host to eliminate the pathogen might result in sepsis.
- The ability of S. agalactiae to induce proinflammatory cytokine production results in the release of tumor necrosis factor-alpha (TNF-α) IL-1 and IL-6, which causes further inflammatory damage to different parts of the body like the brain.
4. Interaction with the host immune system
- Following the entry of bacteria into sterile body sites, the host immune system attempts to clear the infecting organisms mainly by phagocytosis.
- Effective phagocytosis of S. agalactiae relies on opsonization through complement and serotype-specific antibodies.
- Employing different mechanisms, S. agalactiae can impair opsonophagocytosis.
- Capsular polysaccharides inhibit the deposition of complement component C3 and the activation of the alternative pathway.
- Proteins like the beta C protein and CAMP factor bind nonspecifically to the Fc portion of immunoglobulins, presumably rendering antibodies ineffective for opsonization.
- The recruitment of neutrophils to sites of infection through the chemotactic signal of complement component C5a is impaired by cleavage of this molecule through the C5a peptidase of S. agalactiae.
- S. agalactiae can evade phagocytosis as it can survive for more than 24 h inside of macrophages. Despite the lack of catalase activity, S. agalactiae is more resistant to killing through oxygen radicals.
Clinical Manifestations of Streptococcus agalactiae
The most common syndromes due to invasive Group B streptococci disease in adults are bacteremia without a known focus and skin or soft tissue infections. S. agalactiae concurrently with other organisms have also been implicated in pneumonia and under severe conditions causes meningitis in neonates. Another manifestation of GBS disease includes urinary tract infections, especially in pregnant women.
1. Sepsis/ bacteremia
- Sepsis or Bacteremia during S. agalactiae infections is relatively common in adults and more so in neonates.
- The sepsis is characterized by extensive hemodynamic alterations leading to decreased cardiac output, metabolic acidosis, and multiorgan failure.
- In some cases, the bacteremia may lead to the seeding of the cardiac valves and endocarditis.
- Mortality from S. agalactiae endocarditis was relatively high despite medical and surgical interventions with 41% mortality between 1984 and 2004, but now it has dropped down to about 7-8%.
- Bloodstream infection also enables the bacteria to move throughout the body and cause different severe conditions.
2. Pneumonia
- When S. agalactiae reaches the lower respiratory tract through the aspiration of amniotic fluid as well as through bloodstream infections, cases of pneumonia might be observed.
- This usually results in infants with early-onset (occurring within seven days of life) than in infants with late-onset (occurring after 7-27 days).
- Unilateral or bilateral lobar infiltrates, dementia, neurological disease, and tracheoesophageal fistulae are associated with GBS pneumonia.
- Colonization of the airway with GBS is infrequent in patients with cystic fibrosis, but cases of worse clinical outcomes are sporadic.
3. Meningitis
- Meningitis is the predominant clinical finding in cases of late-onset GBS disease. It is uncommon in adults but a common manifestation of late-onset GBS infection in neonates.
- It occurs when the bacteria leave the cerebral capillaries and enter the CSF and initiate an inflammatory cascade in the subarachnoid space.
- Even in infants that survive the acute phase of the GBS meningitis infection, significant cognitive or neurological sequelae are observed, and most of them suffer from neurodevelopmental impairment.
4. Soft-tissue infections
- Skin and soft tissue infections attributed to GBS may manifest as cellulitis, abscesses, foot infection, or decubitus ulcers.
- Besides, acute and chronic osteomyelitis is also common in infants, children, and adults.
- Infection may arise from direct inoculation from overlying skin/soft tissue infection, skin breakdown such as a decubitus ulcer, or via hematogenous seeding.
- Septic arthritis is also observed in some patients with recent prosthetic joints placement. Any joint may be infected, but commonly affected joints include the knee, ankle, and shoulder.
Laboratory Diagnosis of Streptococcus agalactiae
1. Sample collection
- Two sets of samples are collected; one for the detection of carriers and the other for the diagnosis of cases.
- In the case of carriers, vaginal and rectal swabs are taken and sent in Amies medium within four hours of collection.
- In the case of diagnosis, samples like blood, CSF, scabs are collected depending on the clinical presentations of the disease.
2. Morphological, Cultural and Biochemical characteristics
- Direct microscopic observation might be possible in some cases where the presence of Gram-positive cocci arranged in short chains provide the initial basis for identification.
- For culture, enrichment broth is used with antimicrobials like gentamicin or colistin that suppress the growth of other vaginal flora and allow the growth of S. agalactiae.
- On agar media, blood agar can be used on which the appearance of translucent to opaque, glossy, and flat colonies with a narrow zone β-hemolysis indicate the presence of S. agalactiae.
- Other biochemical tests can then be performed to confirm the presence of the bacteria further.
- Tests like Bacitracin test, CAMP test, and PYR test can be performed as the confirmatory test.
3. Immunological test
- Latex agglutination tests can be performed to detect the presence in the cell wall of the antigen group B of Lancefield classification.
- Because S. agalactiae is the only species of Group B, visible agglutinins in the latex agglutination test confirm the presence of S. agalactiae.
- Currently, commercially available Lancefield antigen grouping sera, obtained from many different suppliers, are available that allow rapid differentiation of β-hemolytic streptococci.
4. Automated identification systems
- A variety of products that incorporate batteries of physiologic tests are commercially available for species identification of streptococci.
- These products generally perform well with commonly isolated pathogenic streptococci, such as S. pyogenes and S. agalactiae.
- New commercial systems for the identification of streptococci include the FDA approved Verigene Gram-positive blood culture (BC-GP) nucleic acid test and the FilmArray platform for the direct identification of Streptococcus pathogens from blood culture bottles.
5. Molecular diagnosis
- Confirmatory diagnosis of S. agalactiae can be achieved by comparing the DNA sequences of 16S rRNA genes or selected housekeeping genes with those of appropriate type strains.
- Besides, molecular techniques like PCR and DNA sequencing can also be performed to confirm the presence of S. agalactiae at the molecular level.
- Ribotyping, the analysis of rRNA by restriction fragment length polymorphism, is an alternative method for molecular differentiation of Streptococcus species.
Treatment of Streptococcus agalactiae
- Streptococcus agalactiae are uniformly regarded to be susceptible in vitro to penicillin, although reduced penicillin susceptibility has been detected in isolates. However, penicillin G remains the mainstay of treatment for invasive disease in adults.
- Generally, S. agalactiae is susceptible to other beta-lactam antibiotics, including ampicillin, first-, second-, and third-generation cephalosporins, and carbapenems, although the level of activity varies among different agents.
- In patients who demonstrate an anaphylactic or severe allergy to beta-lactam antimicrobials, alternative therapies include clindamycin, erythromycin, fluoroquinolones, and vancomycin.
- The duration of therapy depends on the clinical presentation. Ten days of therapy is generally acceptable for bacteremia, pneumonia, pyelonephritis, and skin/soft tissue infections.
- Longer durations of treatment are recommended for meningitis (minimum 14 days), and osteomyelitis, endocarditis, and ventriculitis (minimum four weeks).
- In neonates with presumptive early-onset disease, empiric therapy with ampicillin combined with an aminoglycoside is the standard of care.
Prevention of Streptococcus agalactiae
- Due to the increased cases of S. agalactiae disease in neonates, preventative measures have been developed to minimize invasive disease.
- The use of intravenous intrapartum antibiotic prophylaxis to prevent early-onset GBS disease in the infant was first studied in the 1980s and is continued till now.
- Penicillin is the agent of choice for intrapartum antibiotic prophylaxis, with ampicillin as an acceptable alternative.
- An alternative strategy, undergoing development, to prevent neonatal and maternal GBS disease is vaccination of mothers in the third trimester against S. agalactiae.
References
- Topley WWC (2007). Topley and Wison’s Microbiology and Microbial Interactions; Bacteriology, 2 Vol. Tenth Edition. John Wiley and Sons Ltd.
- Bergey, D. H., Whitman, W. B., De, V. P., Garrity, G. M., & Jones, D. (2009). Bergey’s manual of systematic bacteriology: Vol. 3. New York: Springer.
- Raabe, V. N., & Shane, A. L. (2019). Group B Streptococcus(Streptococcus agalactiae). Microbiology spectrum, 7(2), 10.1128/microbiolspec.GPP3-0007-2018. https://doi.org/10.1128/microbiolspec.GPP3-0007-2018
- Spellerberg, B. (2000). Pathogenesis of neonatal Streptococcusagalactiae infections. Microbes and Infection, 2(14), 1733–1742.doi:10.1016/s1286-4579(00)01328-9
- Burnham, C.-A. D., & Tyrrell, G. J. (2003). Virulence factors of group B streptococci. Reviews in Medical Microbiology, 14(4), 109–118.doi:10.1097/00013542-200310000-00002
- Renzo Scanziani, Beatrice Dozio, Ivano Baragetti, Paolo Grillo, Laura Colombo, Sebastiano De Liso, Maurizio Surian, Vaginal colonization with group B Streptococcus (Streptococcus agalactiae) and peritonitis in a woman on CAPD, Nephrology Dialysis Transplantation, Volume 14, Issue 9, September 1999, Pages 2222–2224, https://doi.org/10.1093/ndt/14.9.2222
- Whidbey, C., Harrell, M. I., Burnside, K., Ngo, L., Becraft, A. K., Iyer, L. M., Aravind, L., Hitti, J., Adams Waldorf, K. M., & Rajagopal, L. (2013). A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. The Journal of experimental medicine, 210(6), 1265–1281. https://doi.org/10.1084/jem.20122753
- Shabayek, S., & Spellerberg, B. (2018). Group B Streptococcal Colonization, Molecular Characteristics, and Epidemiology. Frontiers in microbiology, 9, 437. https://doi.org/10.3389/fmicb.2018.00437
- Spellerberg B, Brandt C. Laboratory Diagnosis of Streptococcus pyogenes (group A streptococci) 2016 Feb 10. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes : Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK343617/
- Rosa-Fraile, M., Dramsi, S., & Spellerberg, B. (2014). Group B streptococcal haemolysin and pigment, a tale of twins. FEMS Microbiology Reviews, 38(5), 932–946.
- Vornhagen, J., Adams Waldorf, K. M., & Rajagopal, L. (2017). Perinatal Group B Streptococcal Infections: Virulence Factors, Immunity, and Prevention Strategies. Trends in Microbiology, 25(11), 919–931.doi:10.1016/j.tim.2017.05.013
- Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease. MMWR 2010;59. 10.