- When a particulate or insoluble antigen is mixed with its antibody in the presence of electrolytes at a suitable temperature and pH, the particles are clumped or agglutinated.
- Agglutination reactions are classified as direct, indirect (passive) and reverse passive agglutination reactions.
- Direct Agglutination Test refers to the assays in which the antigen directly agglutinates with the antibody.
- Indirect or passive agglutination involves coating of antigen on the surface of a carrier molecule (e.g. RBC, latex or bentonite), such that the antibody binds to the coated antigen and agglutination takes place on the surface of the carrier molecule. They are also referred to as ‘ particle agglutination test’.
- Reverse passive agglutination test is a special type of particle agglutination test in which the antibody is coated on a carrier molecule which detects antigen in the patient’s serum.
Interesting Science Videos
Latex Agglutination Test
A group of passive agglutination tests carried out by coating either antigen or antibody on an artificial carrier particle, called latex bead are called as latex agglutination test. They may alternatively referred to as latex fixation test and may be:
Latex Agglutination Test (LAT) for Antibody Detection: A Passive Agglutination Test with Antigen bound to the surface of latex beads (polystyrene latex particles; 0.8- 1 μm in diameter).
Latex Agglutination Test (LAT) for Antigen Detection: A Reverse Passive Agglutination Test with Antibody bound to the surface of latex beads.
Objectives of Latex Agglutination Test
To detect microbial and viral infections, autoimmune diseases, hormones, drugs or serum proteins by agglutination reaction of antigen-antibody.
Principle of Latex Agglutination Test
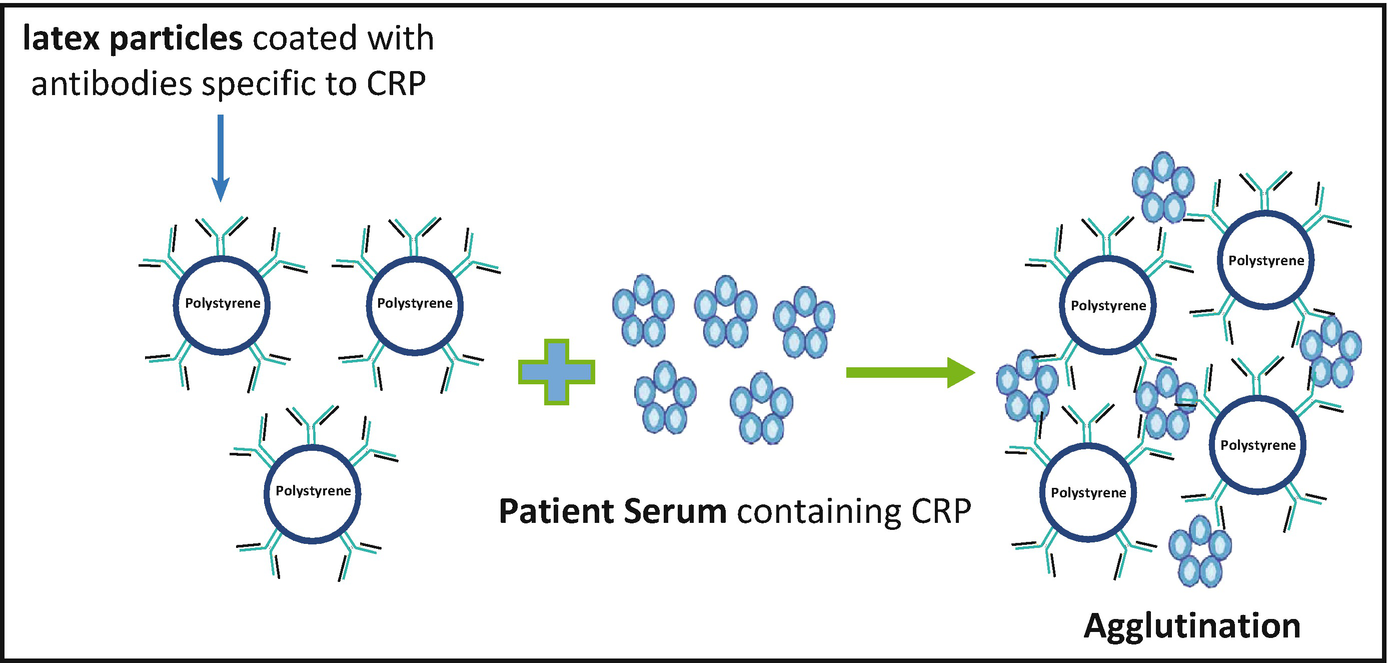
Antibody or antigen molecules can be bound in random alignment to the surface of latex (polystyrene) beads. The number of antibody or antigen molecules bound to each latex particle is large, resulting in a high number of exposed potential binding sites. Antigen or antibody present in a specimen binds to the combining sites of the corresponding antigen/antibody exposed on the surfaces of the latex beads, forming cross-linked aggregates of latex beads and antigen/antibody. Large particle size of latex facilitates the visualization of the antigen-antibody reaction.
Requirements for Latex Agglutination Test
1.5 ml Vials, Microcentrifuge, Pipette, Microtips, Laboratory refrigerator, Glycine saline buffer, Blocking buffer, Antigen for coating, Latex beads, Test antiserum, Glass slides, Beaker, Tooth pick.
Procedure of Latex Agglutination Test
The common procedure for both types of latex test involves coating microbeads of latex with pathogen-specific antigens or antibodies. Patient’s cerebrospinal fluid, serum or urine is mixed with the coated latex particles in serial dilutions with normal saline and observed for agglutination (clumping).
Coating of Latex (For detection of antibodies)
- To 20 μl of latex beads taken in a 1.5 ml vial add 40 μl of glycine-saline buffer.
- Add 60 μl of antigen to the latex and incubate at 37oC for 2 hours.
- Spin down at 5000 rpm for 10 minutes and carefully aspirate the supernatant.
- Resuspend the pellet in 1 ml of blocking buffer and spin down at 5000 rpm for 10 minutes.
- Repeat the washing once more.
- Add 90 μl of blocking buffer to the pellet, mix well.
- Incubate at 4oC, overnight.
Agglutination Test
- To 200 μl of glycine-saline buffer taken in a vial, add 4 μl of test antisera. (50 times diluted).
- Add 50 μl of antigen to 50 μl of diluted antiserum in a 1.5 ml vial, mix well and incubate at room temperature for 10 minutes.
- Pipette 10 μl of coated latex onto a glass slides.
- Add 10 μl of diluted test antiserum to slide A.
- Add 10 μl of antiserum mixed with antigen (from step 1) to B.
- Add 10 μl of glycine-saline buffer to C.
- Take a tooth pick and mix the content in each slide. Discard the tooth pick after using in one slide (take a new one for the next slide).
- After mixing, wait for 2 minutes to observe the result.
Results and Interpretation of Latex Agglutination Test
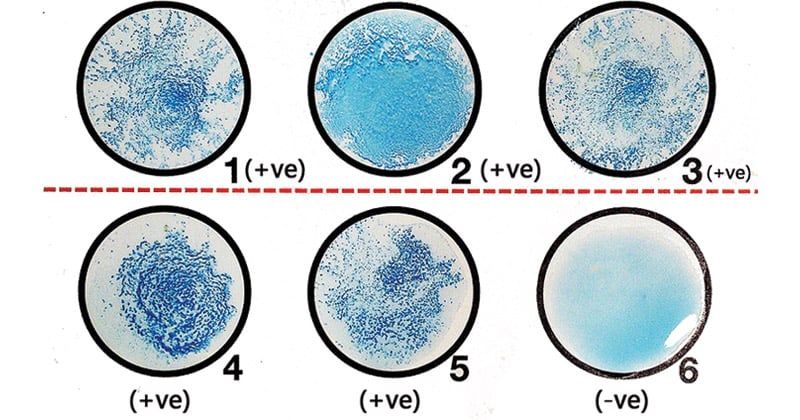
Depending on the procedure, some reactions are reported as positive or negative and other reactions are graded on a 1+ to 4+ scale, with 2+ usually the minimum amount of agglutination visible in a positive sample without the aid of a microscope.
Positive: Agglutination of the beads evident by clumps in any of the dilutions is considered a positive result.
It confirms either that the patient’s body has produced the pathogen-specific antibody (if the test supplied the antigen) or that the specimen contains the pathogen’s antigen (if the test supplied the antibody).
Negative: No agglutination or formation of clumps.
Absence of pathogen-specific antigen or antibody.
Applications of Latex Agglutination Test
- Latex tests are very popular in clinical laboratories for detecting antigen to Cryptococcus neoformans in cerebrospinal fluid or serum. It is also used for detection of capsular antigens of Pneumococcus, Haemophilus influenzae and Meningococcus.
- To confirm the presence of beta-hemolytic Streptococcus from culture plates.
- Latex tests are continually being developed for a variety of organisms such as for the detection of Clostridium difficile toxins A and B, rotavirus, and Escherichia coli 0157:H7 from suspect colonies of E coli.
- Latex agglutination test using latex particles coated with anti-CRP antibodies is the most widely used method employed worldwide for detection of C-reactive protein. Detection limit of CRP by latex agglutination test is 0.6 mg/dl.
- Latex Agglutination Test (LAT) for Antibody Detection is used for detection of ASO (antistreptolysin O antibody).
Advantages of Latex Agglutination Test
- The size of the latex bead (0.8 μm or larger) enhances the ease with which the agglutination reaction is visualized.
- LAT is one of the most widely used tests at present as it is very simple and rapid. It is believed to have replaced many other serological tests including co agglutination tests.
- They are inexpensive, relatively stable and not subject to cross reactivity with other antibodies.
- Levels of bacterial polysaccharides detected by latex agglutination have been shown to be as low as 0.1 ng/mL.
Limitations of Latex Agglutination Test
- Because the pH, osmolarity, and ionic concentration of the solution influence the amount of binding that occurs, conditions under which latex agglutination procedures are carried out must be carefully standardized.
- Some constituents of body fluids, such as rheumatoid factor, have been found to cause false positive reactions in the latex agglutination systems available.
- Some agglutination methods require specimens to be pretreated by heating at 56°C or with ethylenediaminetetraacetic acid (EDTA) before testing making it a tedious process.
References
- http://vlab.amrita.edu/?sub=3&brch=69&sim=195&cnt=2
- https://en.wikipedia.org/wiki/Latex_fixation_test
- Singer JM, Edberg SC, Selinger M, Amram M (1979). “Quality control of the latex-fixation test”. Am. J. Clin. Pathol. 72 (4): 591–6.
- Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers.
- Parija S.C. (2012). Textbook of Microbiology & Immunology.(2 ed.). India: Elsevier India.
- Sastry A.S. & Bhat S.K. (2016). Essentials of Medical Microbiology. New Delhi : Jaypee Brothers Medical Publishers.

Good keep it up