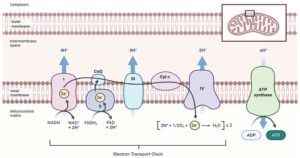
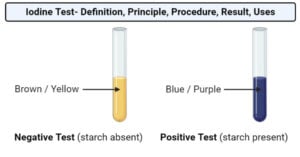
Transcriptomics: Definition, Types, Techniques, Applications
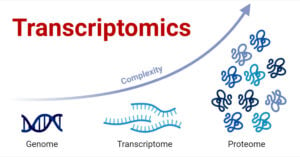
Transcriptomics is the field of science that deals with the study of the transcriptome, which is the collection of all RNA transcripts produced within specific cells of an organism. Transcriptomics … Read more