PYR test (L-Pyrrolidonyl-β-naphthylamide test) is a biochemical test used to detect the ability of bacteria to produce pyrrolidonyl aminopeptidase enzyme using L-Pyrrolidonyl-β-naphthylamide (PYR) as substrate.
It was first reported in 1981 by Godsey J, Schulman R, and Erique LA. They used PYR as a substrate to identify Streptococcus pyogenes and Enterococci.
This test is now used in clinical and research laboratories for diagnostic purposes, mostly for the detection of Streptococcus pyogenes, Enterococcus spp., E. coli, and for the identification of Staphylococcus spp.
There are two methods currently in use for performing the PYR test; the tube method using PYR broth and the disk method using PYR-impregnated disk.
Interesting Science Videos
Objectives
- To assess the ability of bacteria to produce pyrrolidonyl aminopeptidase enzyme
- To biochemically differentiate and presumptively identify sample bacteria
Principle of PYR Test
If bacteria have the ability to synthesize pyrrolidonyl aminopeptidase enzyme, the L-Pyrrolidonyl-β-naphthylamide substrate in the broth or disk is hydrolyzed. This hydrolysis results in the release of β-naphthylamide which reacts with N, N-dimethylaminocinnamaldehyde forming a bright pink or cherry red color Schiff base indicating a positive PYR test.
Requirements for PYR Test
Culture Media
PYR broth is used for the broth (tube) method of the PYR test.
Composition of PYR Broth per 1000 mL
Beef Heart Infusion- 500.00 grams
Peptic Digest of Animal Tissue- 20.00 grams
Dextrose- 2.00 grams
Disodium Phosphate- 0.40 grams
Sodium Chloride- 2.00 grams
Sodium Carbonate- 2.50 grams
Chromogenic Mixture- 0.10 grams
Final pH 7.8±0.2 at 25°C
(References: PYR Broth (himedialabs.com))
(Alternatively, PYR agar medium can be used. PYR agar will have all the components of PYR broth with 15.0 grams of agar powder to make it solidified.)
Preparation of PYR Broth
- Measure the appropriate amount of PYR broth (agar) powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company.
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components dissolve completely in water.
- Dispense about 5 mL (or the required volume) of the medium in test tubes, tighten the cap of the test tubes, or cotton-plug them.
- Autoclave the tubes at 121°C and 15 lbs pressure for 15 minutes and let them cool to around room temperature (below 40°C).
Reagents
PYR Reagent
0.01% p-N, N-dimethylaminocinnamaldehyde (or 1% p-N, N-dimethylaminocinnamaldehyde) is used as a PYR reagent.
Composition of PYR Reagent:
N, N-dimethylaminocinnamaldehyde- 1.0 grams
Concentrated Hydrochloric Acid (HCl)- 1.0 mL
Distilled Water- 99.0 mL
(Reference: PYR Reagent (himedialabs.com))
Preparation of PYR Reagent:
- Add 1.0 grams of N, N-dimethylaminocinnamaldehyde in 99.0 mL of distilled water and slowly add 1.0 mL of concentrated HCl with constant stirring.
- Mix properly so that N, N-dimethylaminocinnamaldehyde dissolves completely.
- Store at 2 to 8°C in a dark place.
PYR Impregnated Disk (for the rapid method or disk test)
Equipment
| Test tubes Forceps | Weighing Machine Autoclave | Bunsen burner Incubator | Inoculating loop Petri Plate |
PPE and other general laboratory materials
Test Organisms (Sample Bacteria)
Positive Control: Streptococcus pyogenes ATCC 19615
Negative Control: E. coli ATCC 25922
Procedure of PYR Test
Tube Method (PYR Broth or Agar Method)
- Using a sterile inoculating loop, pick sample bacteria from a well-isolated colony of fresh culture (18 to 24 hours old culture) and inoculate the PYR broth.
(Alternatively, prepare 0.5 McFarland standards suspension of sample bacteria and transfer a loop full of the suspension to the PYR broth.)
- Incubate aerobically at 35±2°C for about 4 hours (18 to 24 hours for PYR Agar).
- Add 1-2 drops of PYR reagent.
- Observe for color change after 1 to 2 minutes of addition of the reagent.
Rapid Method (PYR Disk Method)
- Place a PYR disk in a sterile petri plate with forceps.
- Moisten the disk with sterile distilled water.
- Using a sterile inoculating loop, pick up 1 or 2 loops full of well-isolated sample bacteria (preferably from Blood Agar Plate: -hemolytic colonies from BAP) and rub them onto the PYR disk.
(Transfer several loops full of bacteria if the sample bacteria is slow-growing bacteria that needs 48 hours or more hours of incubation for growth.)
- Incubate the plate aerobically for 2 minutes at room temperature. (Allow incubation for about 10 minutes for slow-growing bacteria.)
- Add 1 to 2 drops of PYR reagent over the PYR disk.
- Observe for color change after 1 to 2 minutes of addition of the reagent.
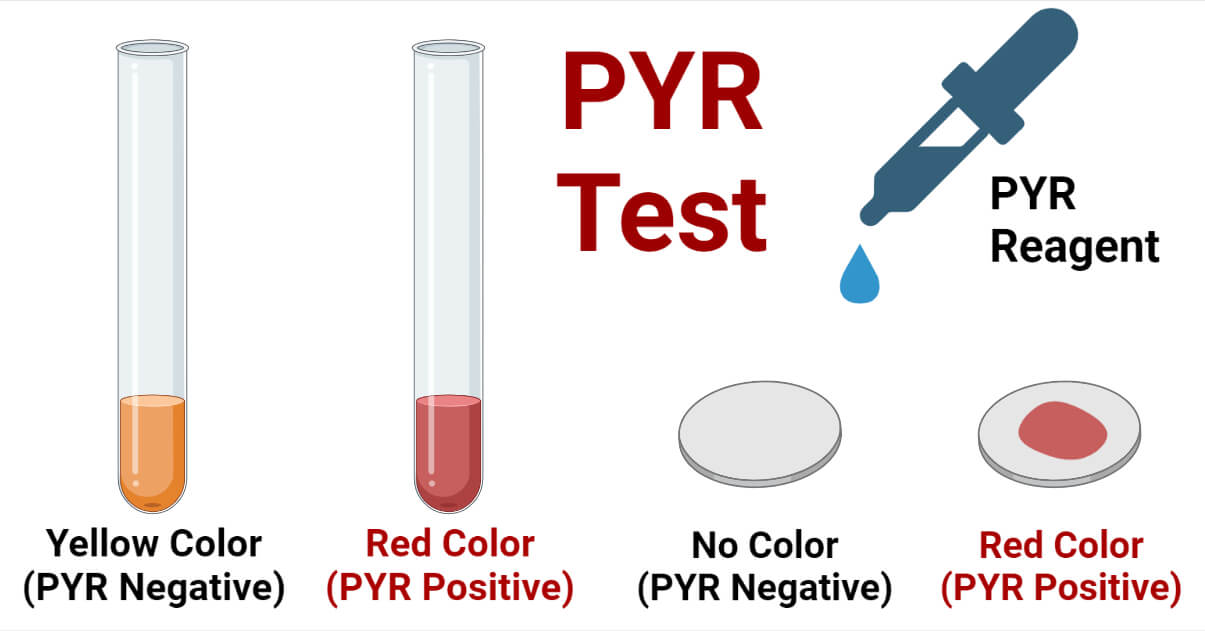
Result and Interpretation of PYR Test
- Development of bright pink or cherry red color over disk or broth (agar) after 1 to 2 minutes of addition of the PYR reagent indicates a positive reaction (PYR positive).
- No color change (or formation of blue color, yellow color, or orange color) after adding the PYR reagent indicates a negative reaction (PYR negative).
- The development of a slight (faint or weak) pink color also indicates a negative reaction.
PYR Positive Bacteria
Streptococcus pyogenes, Enterococcus faecalis, Enterococcus faecium, Citrobacter spp., Klebsiella spp., Yersinia spp., Staphylococcus haemolyticus, etc.
PYR Negative Bacteria
E. coli, Streptococcus agalactiae, Streptococcus bovis, Salmonella spp., Staphylococcus aureus, etc.
Quality Control
Streptococcus pyogenes ATCC 19615 results in the formation of bright pink (red) color on the broth (over the disk or agar) within 1-2 minutes of the addition of the PYR reagent.
E. coli ATCC 25922 doesn’t form pink or red color (doesn’t change color) after the addition of the PYR reagent.
Precautions
- Store PYR reagent in cold and dark conditions.
- Don’t saturate the PYR disk with water. Moisten it slightly.
- Transfer a heavy inoculum and rub it over the disk. Using too little inoculum may give false negative results due to the formation of slight pink color.
- Don’t report faint pink color as a positive reaction.
Applications of PYR Test
- For identification of Streptococcus pyogenes among other -hemolytic Streptococci.
- Differentiation of Group A and Group D Streptococci (PYR positive) from other Streptococci.
- Identification of E. coli among other indole-positive, lactose-positive, Gram-negative bacilli.
- Differentiation of Citrobacter spp. (PYR positive) from other H2S-positive Gram-negative rods; Salmonella spp. (PYR negative).
Limitations of PYR Test
- It is not enough for the complete identification of bacteria; hence, requires other biochemical tests to confirm the identification of the sample bacteria.
- It gives a false negative result if the disk is too moist.
- It gives false negative results if the inocula (sample bacteria) are taken from selective or tube-biochemical test media.
- The use of lower (inadequate) inoculum may give a false negative result.
- Reading results before 1 minute may give unspecific results.
- E. coli and other indole-positive bacteria form blue color over the media and disk, which can be confusing (is regarded as PYR negative).
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- Godsey J, Schulman R, Eriquez LA. 1981. The hydrolysis of L-pyrrolidonylβ-naphthylamide as an aid in the rapid identification of Streptococcus pyogenes, S. avium, and group D enterococci, abstr C84, p 276. Abstr 81st Annu Meet Am Soc Microbiol 1981. American
- PYR Test: Uses, Principle, Procedure and Result Interpretation (universe84a.com)
- PYR Test: Principle, Procedure, Results • Microbe Online
- Remel™ PYR Reagent (thermofisher.com)
- IFU21258.pdf (thermofisher.com)
- PYR Test Principle, Procedure, Result (microbiologynote.com)
- PYR Test- Principle, Uses, Procedure and Result Interpretation (microbiologyinfo.com)
- PYR Test: Result, Principle, Procedure, and Reagents (researchtweet.com)
- PYR Test: Uses, Principle, Procedure and Result Interpretation (universe84a.com)
- PYR Test Principle, Procedure, Result (2023) (ngontinh24.com)
- PYR test – WikiSkripta
