Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
- The Russian botanist Mikhail Tswett coined the term chromatography in 1906.
- The first analytical use of chromatography was described by James and Martin in 1952, for the use of gas chromatography for the analysis of fatty acid mixtures.
- A wide range of chromatographic procedures makes use of differences in size, binding affinities, charge, and other properties to separate materials.
- It is a powerful separation tool that is used in all branches of science and is often the only means of separating components from complex mixtures.
Interesting Science Videos
Principle of Chromatography (how does chromatography work)
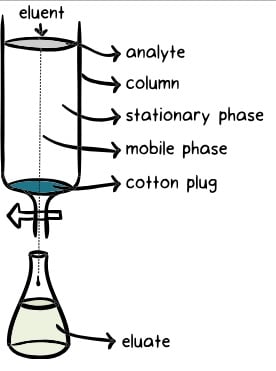
Image Source: Khan Academy
- Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase (stable phase) is separating from each other while moving with the aid of a mobile phase.
- The factors effective on this separation process include molecular characteristics related to adsorption (liquid-solid), partition (liquid-solid), and affinity or differences among their molecular weights.
- Because of these differences, some components of the mixture stay longer in the stationary phase, and they move slowly in the chromatography system, while others pass rapidly into the mobile phase, and leave the system faster.
Three components thus form the basis of the chromatography technique.
- Stationary phase: This phase is always composed of a “solid” phase or “a layer of a liquid adsorbed on the surface solid support”.
- Mobile phase: This phase is always composed of “liquid” or a “gaseous component.”
- Separated molecules
The type of interaction between the stationary phase, mobile phase, and substances contained in the mixture is the basic component effective on the separation of molecules from each other.
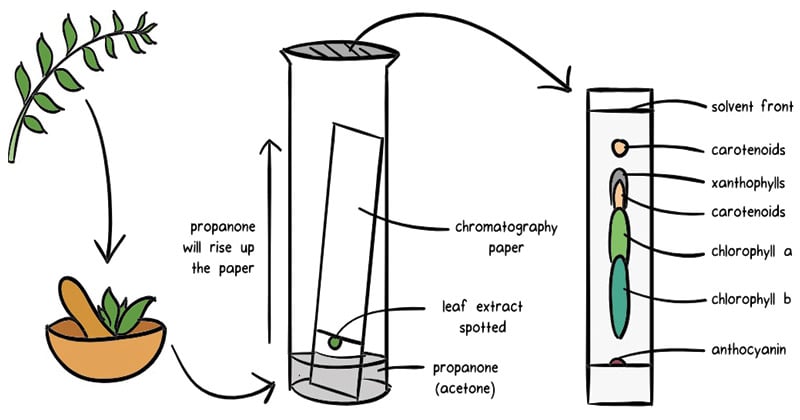
Image Source: Khan Academy
Types of Chromatography
- Substances can be separated on the basis of a variety of methods and the presence of characteristics such as size and shape, total charge, hydrophobic groups present on the surface, and binding capacity with the stationary phase.
- This leads to different types of chromatography techniques, each with their own instrumentation and working principle.
- For instance, four separation techniques based on molecular characteristics and interaction type use mechanisms of ion exchange, surface adsorption, partition, and size exclusion.
- Other chromatography techniques are based on the stationary bed, including column, thin layer, and paper chromatography.
Commonly employed chromatography techniques include:
- Column chromatography
- Ion-exchange chromatography
- Gel-permeation (molecular sieve) chromatography
- Affinity chromatography
- Paper chromatography
- Thin-layer chromatography
- Gas chromatography (GS)
- Dye-ligand chromatography
- Hydrophobic interaction chromatography
- Pseudoaffinity chromatography
- High-pressure liquid chromatography (HPLC)
Applications of Chromatography
Pharmaceutical sector
- To identify and analyze samples for the presence of trace elements or chemicals.
- Separation of compounds based on their molecular weight and element composition.
- Detects the unknown compounds and purity of mixture.
- In drug development.
Chemical industry
- In testing water samples and also checks air quality.
- HPLC and GC are very much used for detecting various contaminants such as polychlorinated biphenyl (PCBs) in pesticides and oils.
- In various life sciences applications
Food Industry
- In food spoilage and additive detection
- Determining the nutritional quality of food
Forensic Science
- In forensic pathology and crime scene testing like analyzing blood and hair samples of crime place.
Molecular Biology Studies
- Various hyphenated techniques in chromatography such as EC-LC-MS are applied in the study of metabolomics and proteomics along with nucleic acid research.
- HPLC is used in Protein Separation like Insulin Purification, Plasma Fractionation, and Enzyme Purification and also in various departments like Fuel Industry, biotechnology, and biochemical processes.
References
- https://chromatography.conferenceseries.com/events-list/applications-of-chromatography
- http://www.biologydiscussion.com/biochemistry/chromatography-techniques/top-12-types-of-chromatographic-techniques-biochemistry/12730
- http://library.umac.mo/ebooks/b28050630.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5206469/

Excellent!
Very helpful, thanks so much 8
Because mobile phase travels against gravity so the gravitational force on it causes it to slow down
Osm article …. Thanks
Thanks for this article…. It was excellent..,..
Thanks you
Thank You So Much. Needed Info For A Project And Got It All Here!
Very good , i like it very much
Why is the rate of movement of mobile phase less in ascending Paper Chromatography?
It is because of the molecular weight. Components with large molecular weight rise up slowly.
Excellent article really like it
excellence article related to chromatography.brief revision of chromatography. thanks all team.
Excellent article.
Why is the rate of movement of mobile phase less in ascending Paper Chromatography?
What is Chromatogram?