Interesting Science Videos
What is Paper Chromatography?
Paper chromatography (PC) is a type of planar chromatography whereby chromatography procedures are run on a specialized paper.
PC is considered to be the simplest and most widely used of the chromatographic techniques because of its applicability to isolation, identification, and quantitative determination of organic and inorganic compounds.
It was first introduced by German scientist Christian Friedrich Schonbein (1865).
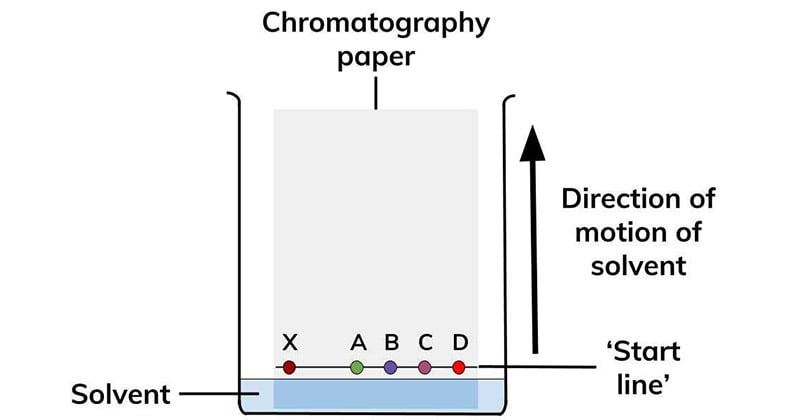
Types of Paper chromatography
Paper Adsorption Chromatography
Paper impregnated with silica or alumina acts as adsorbent (stationary phase) and solvent as mobile phase.
Paper Partition Chromatography
Moisture / Water present in the pores of cellulose fibers present in filter paper acts as stationary phase & another mobile phase is used as solvent In general paper chromatography mostly refers to paper partition chromatography.
Principle of Paper chromatography
The principle of separation is mainly partition rather than adsorption. Substances are distributed between a stationary phase and a mobile phase. Cellulose layers in filter paper contain moisture which acts as a stationary phase. Organic solvents/buffers are used as mobile phase. The developing solution travels up the stationary phase carrying the sample with it. Components of the sample will separate readily according to how strongly they adsorb onto the stationary phase versus how readily they dissolve in the mobile phase.
Instrumentation of Paper chromatography
- Stationary phase & papers used
- Mobile phase
- Developing Chamber
- Detecting or Visualizing agents
1. STATIONARY PHASE AND PAPERS
- Whatman filter papers of different grades like No.1, No.2, No.3, No.4, No.20, No.40, No.42 etc
- In general the paper contains 98-99% of α-cellulose, 0.3 – 1% β -cellulose.
Other modified papers
- Acid or base washed filter paper
- Glass fiber type paper.
- Hydrophilic Papers – Papers modified with methanol, formamide, glycol, glycerol etc.
- Hydrophobic papers – acetylation of OH groups leads to hydrophobic nature, hence can be used for reverse phase chromatography.
- Impregnation of silica, alumna, or ion exchange resins can also be made.
2. PAPER CHROMATOGRAPHY MOBILE PHASE
- Pure solvents, buffer solutions or mixture of solvents can be used.
Examples-
Hydrophilic mobile phase
- Isopropanol: ammonia:water 9:1:2
- Methanol : water 4:1
- N-butanol : glacial acetic acid : water 4:1:5
Hydrophobic mobile phases
- dimethyl ether: cyclohexane kerosene : 70% isopropanol
- The commonly employed solvents are the polar solvents, but the choice depends on the nature of the substance to be separated.
- If pure solvents do not give satisfactory separation, a mixture of solvents of suitable polarity may be applied.
3. CHROMATOGRAPHIC CHAMBER
- The chromatographic chambers are made up of many materials like glass, plastic or stainless steel. Glass tanks are preferred most.
- They are available invarious dimensional size depending upon paper length and development type.
- The chamber atmosphere should be saturated with solvent vapor.
Steps in Paper Chromatography
In paper chromatography, the sample mixture is applied to a piece of filter paper, the edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action. The basic steps include:
Selection of Solid Support
Fine quality cellulose paper with defined porosity, high resolution, negligible diffusion of the sample, and favoring good rate of movement of solvent.
Selection of Mobile Phase
Different combinations of organic and inorganic solvents may be used depending on the analyte.
Example. Butanol: Acetic acid: Water (12:3:5) is a suitable solvent for separating amino acids.
Saturation of Tank
The inner wall of the tank is wrapped with filter paper before the solvent is placed in the tank to achieve better resolution.
Sample Preparation and Loading
If the solid sample is used, it is dissolved in a suitable solvent. Sample (2-20ul) is added on the baseline as a spot using a micropipette and air dried to prevent the diffusion.
Development of the Chromatogram
Sample loaded filter paper is dipped carefully into the solvent not more than a height of 1 cm and waited until the solvent front reaches near the edge of the paper.
Different types of development techniques can be used:
ASCENDING DEVELOPMENT
- Like conventional type, the solvent flows against gravity.
- The spots are kept at the bottom portion of paper and kept in a chamber with mobile phase solvent at the bottom.
DESCENDING TYPE
- This is carried out in a special chamber where the solvent holder is at the top.
- The spot is kept at the top and the solvent flows down the paper.
- In this method solvent moves from top to bottom so it is called descending chromatography.
ASCENDING – DESCENDING DEVELOPMENT
- A hybrid of above two techniques is called ascending-descending chromatography.
- Only length of separation increased, first ascending takes place followed by descending.
CIRCULAR / RADIAL DEVELOPMENT
- Spot is kept at the centre of a circular paper.
- The solvent flows through a wick at the centre & spreads in all directions uniformly.
Drying of Chromatogram
After the development, the solvent front is marked and left to dry in a dry cabinet or oven.
Detection
Colorless analytes were detected by staining with reagents such as iodine vapor, ninhydrin, etc.
Radiolabeled and fluorescently labeled analytes were detected by measuring radioactivity and fluorescence respectively.
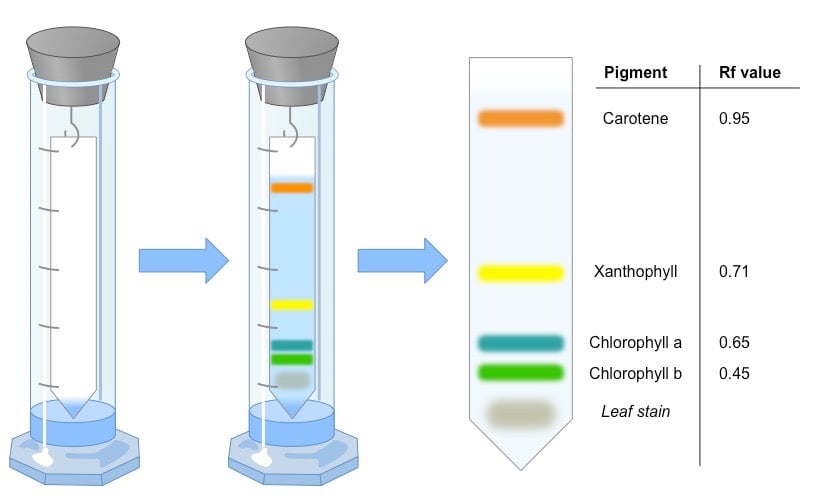
Rf values
Some compounds in a mixture travel almost as far as the solvent does; some stay much closer to the baseline. The distance traveled relative to the solvent is a constant for a particular compound as long as other parameters such as the type of paper and the exact composition of the solvent are constant. The distance traveled relative to the solvent is called the Rf value.
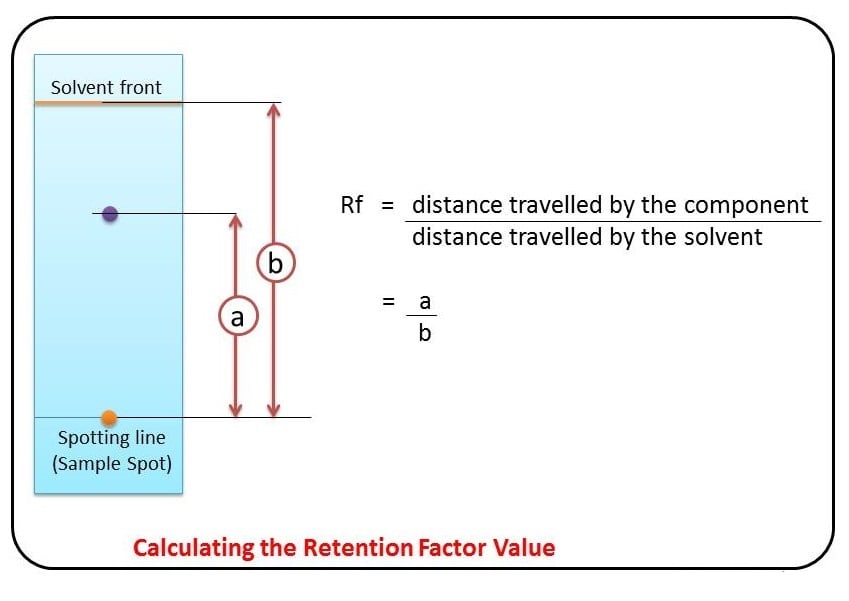
Thus, in order to obtain a measure of the extent of movement of a component in a paper chromatography experiment, “Rf value” is calculated for each separated component in the developed chromatogram. An Rf value is a number that is defined as the distance traveled by the component from the application point.
Applications of Paper Chromatography
- To check the control of purity of pharmaceuticals,
- For detection of adulterants,
- Detect the contaminants in foods and drinks,
- In the study of ripening and fermentation,
- For the detection of drugs and dopes in animals & humans
- In analysis of cosmetics
- Analysis of the reaction mixtures in biochemical labs.
Advantages of Paper Chromatography
- Simple
- Rapid
- Paper Chromatography requires very less quantitative material.
- Paper Chromatography is cheaper compared to other chromatography methods.
- Both unknown inorganic as well as organic compounds can be identified by paper chromatography method.
- Paper chromatography does not occupy much space compared to other analytical methods or equipments.
- Excellent resolving power
Limitations of Paper Chromatography
- Large quantity of sample cannot be applied on paper chromatography.
- In quantitative analysis paper chromatography is not effective.
- Complex mixture cannot be separated by paper chromatography.
- Less Accurate compared to HPLC or HPTLC
References
- http://frndzzz.com/Advantages-and-Disadvantages-of-Paper-Chromatography
- https://www.slideshare.net/shaisejacob/paper-chromatography-pptnew?next_slideshow=1
- https://www.slideshare.net/shaisejacob/paper-chromatography-ppt-new
- https://www.biochemden.com/paper-chromatography/
- http://web.engr.oregonstate.edu/~rochefow/K-12%20Outreach%20Activities/Microfluidics%20&%20Pregnancy%20Test%20Kit%20Lab/paper%20chromatography_Chemguide.pdf
- https://pubs.acs.org/doi/abs/10.1021/ac60051a002

Examples of substances that can be effectively separated by paper chromatography is necessary.
I enjoy this write up, but the definition of Rf Values just mention distance traveled by the solute from the point from the point of application of the sample, how about the total distance traveled by the solvent?
Some examples of how Rf value can be calculated is necessary.
Pls how is charging a disadvantage of filter paper in chromatography
Hi! I enjoyed reading it. Hmm just wanna know somepoints.
Why it is best to use the farthest distance traveled by a sugar if and when the solvent went over the paper and what is the purpose of heating the chromatographic paper after running the procedure?
This links are so much useful and it’s very helpful also….
Thanks for sharing important details like this. I enjoyed reading your site, and love to know the latest updates.