Lipids are a group of diverse macromolecules consisting of fatty acids and their derivatives that are insoluble in water but soluble in organic solvents.
- Lipids consist of fats, oils, hormones, and certain components of membranes that are grouped together because of their hydrophobic interactions.
- The lipids are essential constituents of the diet because of their high energy value.
- These are also essential for the fat-soluble vitamins and the essential fatty acids found with the fat of the natural foodstuffs.
- Fats combined with proteins (lipoproteins) are essential constituents of the cell membranes and mitochondria of the cell.
- Lipids occur naturally in living beings like plants, animals, and microorganisms that form various components like cell membranes, hormones, and energy storage molecules.
- Lipids exist in either liquid or non-crystalline solids at room temperatures and are colorless, odorless, and tasteless.
- These are composed of fatty acids and glycerol.
Interesting Science Videos
Properties of Lipids
- Lipids may be either liquids or non-crystalline solids at room temperature.
- Pure fats and oils are colorless, odorless, and tasteless.
- They are energy-rich organic molecules
- Insoluble in water
- Soluble in organic solvents like alcohol, chloroform, acetone, benzene, etc.
- No ionic charges
- Solid triglycerols (Fats) have high proportions of saturated fatty acids.
- Liquid triglycerols (Oils) have high proportions of unsaturated fatty acids.
1. Hydrolysis of triglycerols
Triglycerols like any other esters react with water to form their carboxylic acid and alcohol– a process known as hydrolysis.
2. Saponification:
Triacylglycerols may be hydrolyzed by several procedures, the most common of which utilizes alkali or enzymes called lipases. Alkaline hydrolysis is termed saponification because one of the products of the hydrolysis is a soap, generally sodium or potassium salts of fatty acids.
3. Hydrogenation
The carbon-carbon double bonds in unsaturated fatty acids can be hydrogenated by reacting with hydrogen to produce saturated fatty acids.
4. Halogenation
Unsaturated fatty acids, whether they are free or combined as esters in fats and oils, react with halogens by addition at the double bond(s). The reaction results in the decolorization of the halogen solution.
5. Rancidity:
The term rancid is applied to any fat or oil that develops a disagreeable odor. Hydrolysis and oxidation reactions are responsible for causing rancidity. Oxidative rancidity occurs in triacylglycerols containing unsaturated fatty acids.
Structure of Lipids
- Lipids are made of the elements Carbon, Hydrogen and Oxygen, but have a much lower proportion of water than other molecules such as carbohydrates.
- Unlike polysaccharides and proteins, lipids are not polymers—they lack a repeating monomeric unit.
- They are made from two molecules: Glycerol and Fatty Acids.
- A glycerol molecule is made up of three carbon atoms with a hydroxyl group attached to it and hydrogen atoms occupying the remaining positions.
- Fatty acids consist of an acid group at one end of the molecule and a hydrocarbon chain, which is usually denoted by the letter ‘R’.
- They may be saturated or unsaturated.
- A fatty acid is saturated if every possible bond is made with a Hydrogen atom, such that there exist no C=C bonds.
- Unsaturated fatty acids, on the other hand, do contain C=C bonds. Monounsaturated fatty acids have one C=C bond, and polyunsaturated have more than one C=C bond.
Classification of Lipids
Lipids can be classified according to their hydrolysis products and according to similarities in their molecular structures. Three major subclasses are recognized:
1. Simple lipids
(a) Fats and oils which yield fatty acids and glycerol upon hydrolysis.
(b) Waxes, which yield fatty acids and long-chain alcohols upon hydrolysis.
Fats and Oils
- Both types of compounds are called triacylglycerols because they are esters composed of three fatty acids joined to glycerol, trihydroxy alcohol.
- The difference is on the basis of their physical states at room temperature. It is customary to call a lipid a fat if it is solid at 25°C, and oil if it is a liquid at the same temperature.
- These differences in melting points reflect differences in the degree of unsaturation of the constituent fatty acids.
Waxes
- Wax is an ester of long-chain alcohol (usually mono-hydroxy) and a fatty acid.
- The acids and alcohols normally found in waxes have chains of the order of 12-34 carbon atoms in length.
2. Compound lipids
(a) Phospholipids, which yield fatty acids, glycerol, amino alcohol sphingosine, phosphoric acid and nitrogen-containing alcohol upon hydrolysis.
They may be glycerophospholipids or sphingophospholipid depending upon the alcohol group present (glycerol or sphingosine).
(b) Glycolipids, which yield fatty acids, sphingosine or glycerol, and a carbohydrate upon hydrolysis.
They may also be glyceroglycolipids or sphingoglycolipid depending upon the alcohol group present (glycerol or sphingosine).
3. Derived lipids:
Hydrolysis product of simple and compound lipids is called derived lipids. They include fatty acid, glycerol, sphingosine and steroid derivatives.
Steroid derivatives are phenanthrene structures that are quite different from lipids made up of fatty acids.
Alcohols and Esters
- The most important and frequently occurring alcohol found in lipids is glycerol. Glycerol is a small organic molecule consisting of three hydroxyls (OH-) groups.
- Glycerol makes up simple lipids which are esters of fatty acids and glycerol and similar alcohols.
- The alcohol might be glycerol or other long-chain alcohol. The long-chain alcohols are mostly mono-hydroxy with a single OH group.
- Depending on the alcohol used, simple lipids consist of fats, oil, or waxes. Fats and oils are esters of fatty acids and glycerol, whereas waxes are esters of fatty acids and long-chain alcohols.
- The esters of fatty acids are formed after the dehydration reaction between the fatty acids and the alcohol molecules.
Triglycerides
Triglycerides are a type of lipid which is an ester of three fatty acids with glycerol. Triglycerides are the main constituents of body fat in humans, other vertebrates, and vegetable fats.
Structure of Triglycerides
Triglycerides are tri-esters where three fatty acid molecules are bound to a single glycerol molecule by covalent ester bonds.
Reaction
HOCH2CH(OH)CH2OH + RCO2H + R′CO2H + R″CO2H → RCO2CH2CH(O2CR′)CH2CO2R″ + 3H2O
- The three fatty acids involved in the condensation reaction are usually different, and their chain length also differs from one another.
- In naturally occurring triglycerides, the fatty acid chains mostly contain 16, 18, or 20 carbon atoms.
- Even-numbered carbon atoms present in animals and plants indicating the pathway of their biosynthesis from two-carbon acetyl CoA.
- Simple triglycerides might also have identical fatty acids forming homotriglycerides.
- The charges in triglycerides are evenly distributed around the molecules, which prevents the formation of hydrogen bonds with water molecules, making them insoluble in water.

Functions of Triglycerides
- Triglycerides are important macromolecules as they store most of the energy in the body.
- These are stored in fat cells which are then released into the bloodstream by the action of different hormones whenever necessary.
- The fat stored in the body forms a layer of insulation beneath the skin, which helps to maintain the body temperature.
- Triglycerides also aid in the absorption and transport of fat-soluble vitamins in the body.
What are Fatty acids?
- Fatty acids are organic molecules that are long-chained carboxylic acids with 4-36 carbon atoms.
- The hydrocarbon chains are either saturated or unsaturated, depending on the bonds between the carbon atoms. If all the carbon-carbon bonds are single, the acid is saturated; if one or more carbon-carbon double bonds are present, the acid is unsaturated.
- Naturally occurring fatty acids are mostly unbranched, and these occur in three main classes of lipids; triglycerides, phospholipids, and cholesteryl esters.
- Fatty acids are not found in the free state but remain associated with alcohol to form triglycerides.
- Fatty acids are stored as an energy reserve (fat) through an ester linkage to glycerol to form triglycerides.
Saturated and Unsaturated Fatty acids
1. Saturated fatty acids
- Saturated fatty acids are the simplest form of fats that are unbranched linear chains of CH2 groups linked together by carbon-carbon single bonds with a terminal carboxylic acid.
- The term ‘saturated’ is used to indicate that the maximum number of hydrogen atoms are bonded to each carbon atom in a molecule of fat.
- The general formula for these acids is CnH2n+1COOH.
- Fatty acids obtained from an animal source are mostly even-numbered linear chains of saturated fatty acids.
- Saturated fatty acids usually have a higher melting point than their counterparts which is why saturated fatty acids remain in the solid-state at room temperatures.
- These are mostly solid and are found in animal fat like butter, meat, and whole milk. But some saturated fatty acids are also found in vegetable sources like vegetable oil, coconut oil, and peanut oil.
2. Unsaturated fatty acids
- Unsaturated fatty acids are more complex fatty acids with bent hydrocarbon chains linked together by one or more carbon-carbon double bonds with a terminal carboxylic acids group.
- The term ‘unsaturated’ indicates that the carbons atoms do not have the maximum possible hydrogen atoms bound to carbon atoms.
- Due to the presence of double bonds, the cis and trans conformation of these molecules are important. The unsaturated fatty acids found in the human body exist in the cis conformation
- Unsaturated fatty acids have a lower melting point as compared to saturated fatty acids, and thus they exist in the liquid state at room temperatures
- Most vegetable oils and fish oils are some of the important sources of unsaturated fatty acids.
Read Also: 20 Differences Between Saturated and Unsaturated fatty acids
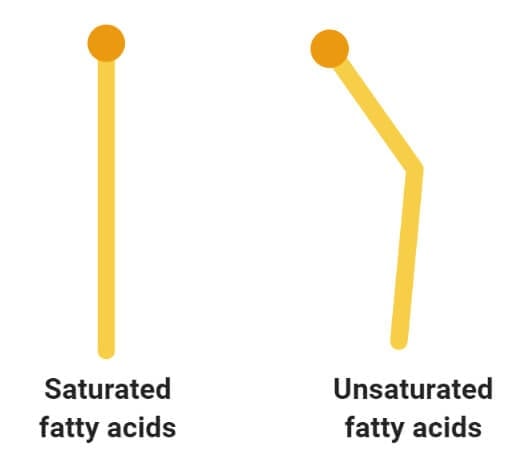
Glycerol and the formation of ester bonds
Image Source: Wikipedia.
- Glycerol is a simple organic compound in three hydroxyl groups that is a colorless, odorless, and viscous liquid.
- It forms the backbone of many lipids that are termed glycerides. The fat is later hydrolyzed into fatty acids and glycerol where the fatty acid provides energy to the body, whereas the glycerol is converted into glucose.
- The reaction involved in the formation of ester bonds is termed as condensation reaction where the free hydroxyl end of the glycerol molecule joins to the OH of the COOH group of the fatty acid.
- The process of condensation is termed esterification due to the formation of ester bonds between the two molecules.
- The lipid molecules formed from three fatty acids and a single glycerol molecule are termed as triacylglycerols or triglycerides.
Phospholipids
- A phospholipid is an organic molecule consisting of fatty acids, a phosphate group, and a glycerol group that forms the main component of various cellular membranes.
- Phospholipid bilayer forms an important part of the cell membrane for the selective transport of molecules in and out of the cell.
- The phosphate group forms the hydrophilic head, whereas the fatty acids form the hydrophobic tails. The head and tail regions in phospholipids are joined by a glycerol molecule.
- The hydrophobic and hydrophilic interaction between different molecules and the lipid bilayer enables the passage of biomolecules. These interactions make the cell membrane amphipathic.
1. Hydrophilic (polar) phosphate heads
- The hydrophilic head or water-loving part of the phospholipids contains a negatively charged phosphate group with an unidentified alkyl group.
- The hydrophilic region might or might not be polar or charged.
- The heads of the phospholipid membrane face outwards that remain in interaction with the aqueous solution inside and outside the cell.
- As water is a polar molecule, the hydrophilic head immediately forms electrostatic interaction with the water molecule.
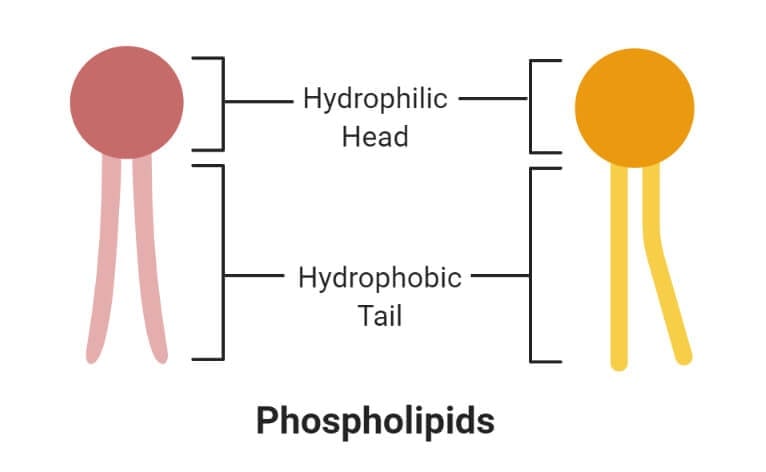
2. Hydrophobic (non-polar) fatty acid tails
- The hydrophobic part of the phospholipid bilayer is also termed the water-fearing portion that consists of long non-polar fatty acid tails.
- These tails easily interact with other hydrophobic molecules but do not interact with water molecules.
- The tail region is a non-polar end where charge-less molecules are present.
- The hydrophobic tails are thus tucked towards the interior of the membrane in order to shield the tails from the surrounding water. This arrangement is also energetically favorable.
- The hydrophobic interactions form a good barrier between the inside and outside of the cell as water, and other charge molecules cannot easily cross the hydrophobic core of the membrane.
Sterols (Cholesterol)
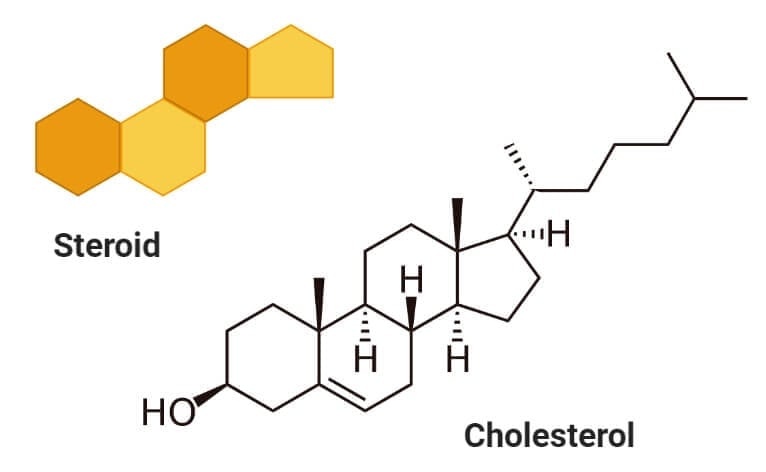
- Sterols are a type of lipids composed of steroid alcohols occurring naturally in plants, animals, fungi, and several bacteria.
- The most important and familiar type of sterol is cholesterol which plays an essential role in cell membrane structure and functions.
- Cholesterol acts as a precursor to fat-soluble vitamins like Vitamin D and hormones.
- Cholesterol is formed of four linked hydrocarbon rings forming the bulk of the steroid structure. One end of cholesterol consists of a hydrocarbon tail, whereas the other end is linked to an alcohol group.
- The hydroxyl group joins with other hydroxyl groups or carbonyl oxygen of phospholipids.
- Cholesterol can be biosynthesized within the body of various animals. In humans, the liver makes up 100% of all cholesterol required for the body.
- Cholesterol is considered essential for the regulation of membrane fluidity in animals. It also increases the permeability of the cell membrane to sodium and potassium ions.
- However, if the concentration of cholesterol increases beyond normal, it might combine with other components in the blood and form plaque. The plaque might attach to the walls of arteries and veins, resulting in coronary artery disease.
Functions of Lipids
- Biological lipids are a chemically diverse group of compounds, and the biological functions of the lipids are as diverse as their chemistry.
- In the body, fats serve as an efficient source of energy and are also stored in the adipose tissues. These also serve as an insulating material in the subcutaneous tissues and around certain organs.
- Phospholipids and sterols are major structural elements of biological membranes.
- Similarly, fats combined with proteins (lipoproteins) are important constituents of the cell membranes and mitochondria of the cell.
- Lipids also act as the structural component of the cell and provide the hydrophobic barrier that allows the separation of the aqueous contents of the cell and subcellular structures.
- Other lipids, although present in relatively small quantities, play crucial roles as enzyme cofactors, electron carriers, light-absorbing pigments, and hydrophobic anchors for proteins.
- Cholesterol acts as a precursor to fat-soluble vitamins like Vitamin D and hormones.
- Lipids are also activators of enzymes like glucose-6-phosphatase, β-hydroxybutyric dehydrogenase, and stearyl CoA desaturase.
References and Sources
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition.
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company.
- Biologydictionary.net Editors. (2016, November 08). Phospholipid. Retrieved from https://biologydictionary.net/phospholipid/
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- 3% – http://ndl.ethernet.edu.et/bitstream/123456789/78706/12/Chap-12.pdf
- 2% – http://fac.ksu.edu.sa/sites/default/files/4-bch302_lipids_i_0.pdf
- 1% – https://www.britannica.com/science/lipid
- 1% – https://noahstrength.com/health/three-kinds-of-triglycerides/
- 1% – https://letslearnplants.blogspot.com/
- 1% – https://ibiologia.com/phospholipid-bilayer/
- 1% – https://hyperleap.com/topic/Cholesterol
- 1% – https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/27%3A_Biomolecules_-_Lipids/27.03%3A_Waxes_Fats_and_Oils
- 1% – https://biologydictionary.net/hydrophilic/
- 1% – http://www.bioinfo.org.cn/book/biochemistry/chapt09/sim1.htm

These notes come in handy. Really really appreciate this effort of combining all these. Thankyou. Very informative material, and such precise and to the point written.
This is astounding introductory notes for lipids under biochemistry, I really enjoyed the part where the concepts of lipid biomolecule connected to chemistry bonds and other stuff like the condensation
Its nice