Gas chromatography differs from other forms of chromatography in that the mobile phase is a gas and the components are separated as vapors.
- It is thus used to separate and detect small molecular weight compounds in the gas phase.
- The sample is either a gas or a liquid that is vaporized in the injection port. The mobile phase for gas chromatography is a carrier gas, typically helium because of its low molecular weight and being chemically inert.
- The pressure is applied and the mobile phase moves the analyte through the column. The separation is accomplished using a column coated with a stationary phase.
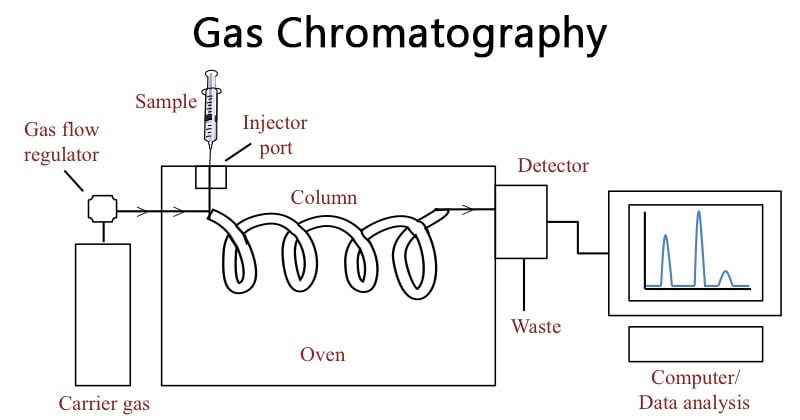
Image Source: Bitesize Bio.
Interesting Science Videos
Principle of Gas chromatography (how does gas chromatography work)
The equilibrium for gas chromatography is partitioning, and the components of the sample will partition (i.e. distribute) between the two phases: the stationary phase and the mobile phase.
Compounds that have a greater affinity for the stationary phase spend more time in the column and thus elute later and have a longer retention time (Rt) than samples that have a higher affinity for the mobile phase.
Affinity for the stationary phase is driven mainly by intermolecular interactions and the polarity of the stationary phase can be chosen to maximize interactions and thus the separation.
Ideal peaks are Gaussian distributions and symmetrical, because of the random nature of the analyte interactions with the column.
- The separation is hence accomplished by partitioning the sample between the gas and a thin layer of a nonvolatile liquid held on a solid support.
- A sample containing the solutes is injected into a heated block where it is immediately vaporized and swept as a plug of vapor by the carrier gas stream into the column inlet.
- The solutes are adsorbed by the stationary phase and then desorbed by a fresh carrier gas.
- The process is repeated in each plate as the sample is moved toward the outlet.
- Each solute will travel at its own rate through the column.
- Their bands will separate into distinct zones depending on the partition coefficients, and band spreading.
- The solutes are eluted one after another in the increasing order of their kd, and enter into a detector attached to the exit end of the column.
- Here they register a series of signals resulting from concentration changes and rates of elution on the recorder as a plot of time versus the composition of carrier gas stream.
- The appearance time, height, width, and area of these peaks can be measured to yield quantitative data.
Parts of Gas chromatography

Gas chromatography is mainly composed of the following parts:
- Carrier gas in a high-pressure cylinder with attendant pressure regulators and flow meters
- Helium, N2, H, Argon are used as carrier gases.
- Helium is preferred for thermal conductivity detectors because of its high thermal conductivity relative to that of most organic vapors.
- N2 is preferable when a large consumption of carrier gas is employed.
- Carrier gas from the tank passes through a toggle valve, a flow meter, (1-1000 ml/min), capillary restrictors, and a pressure gauge (1-4 atm).
- Flow rate is adjusted by means of a needle valve mounted on the base of the flow meter and controlled by capillary restrictors.
- The operating efficiency of the gas chromatograph is directly dependant on the maintenance of constant gas flow.
- Sample injection system
- Liquid samples are injected by a microsyringe with a needle inserted through a self-scaling, silicon-rubber septum into a heated metal block by a resistance heater.
- Gaseous samples are injected by a gas-tight syringe or through a by-pass loop and valves.
- Typical sample volumes range from 0.1 to 0.2 ml.
- The separation column
- The heart of the gas chromatography is the column which is made of metals bent in U shape or coiled into an open spiral or a flat pancake shape.
- Copper is useful up to 2500
- Swege lock fittings make column insertion easy.
- Several sizes of columns are used depending upon the requirements.
- Liquid phases
- An infinite variety of liquid phases are available limited only by their volatility, thermal stability and ability to wet the support.
- No single phase will serve for all separation problems at all temperatures.
Non-Polar – Parafin, squalane, silicone greases, apiezon L, silicone gum rubber. These materials separate the components in order of their boiling points.
Intermediate Polarity – These materials contain a polar or polarizable group on a long non-polar skeleton which can dissolve both polar and non-polar solutes. For example. diethyl hexyl phthalate is used for the separation of high boiling alcohols.
Polar – Carbowaxes – Liquid phases with a large proportion of polar groups. Separation of polar and non-polar substances.
Hydrogen bonding – Polar liquid phases with high hydrogen bonding e.g. Glycol.
Specific purpose phases – Relying on a chemical reaction with solute to achieve separations. e.g AgNO3 in glycol separates unsaturated hydrocarbons.
- Supports
- The structure and surface characteristics of the support materials are important parameters, which determine the efficiency of the support and the degree of separation respectively.
- The support should be inert but capable of immobilizing a large volume of liquid phase as a thin film over its surface.
- The surface area should be large to ensure the rapid attainment of equilibrium between stationary and mobile phases.
- Support should be strong enough to resist breakdown in handling and be capable of packed into a uniform bed.
- Diatomaceous earth, kieselguhr treated with Na 2CO 3 for 9000 C causes the particle fusion into coarser aggregates.
- Glass beads with a low surface area and low porosity can be used to coat up to 3% stationary phases.
- Porous polymer beads differing in the degree of cross-linking of styrene with alkyl-vinyl benzene are also used which are stable up to 2500
- Detector
- Detectors sense the arrival of the separated components and provide a signal.
- These are either concentration-dependent or mass dependant.
- The detector should be close to the column exit and the correct temperature to prevent decomposition.
- Recorder
- The recorder should be generally 10 mv (full scale) fitted with a fast response pen (1 sec or less). The recorder should be connected with a series of good quality resistances connected across the input to attenuate the large signals.
- An integrator may be a good addition.
The procedure of Gas Chromatography
Step 1: Sample Injection and Vapourization
- A small amount of liquid sample to be analyzed is drawn up into a syringe.
- The syringe needle is positioned in the hot injection port of the gas chromatograph and the sample is injected quickly.
- The injection of the sample is considered to be a “point” in time, that is, it is assumed that the entire sample enters the gas chromatograph at the same time, so the sample must be injected quickly.
- The temperature is set to be higher than the boiling points of the components of the mixture so that the components will vaporize.
- The vaporized components then mix with the inert gas mobile phase to be carried to the gas chromatography column to be separated.
Step 2: Separation in the Column
- Components in the mixture are separated based on their abilities to adsorb on or bind to, the stationary phase.
- A component that adsorbs most strongly to the stationary phase will spend the most time in the column (will be retained in the column for the longest time) and will, therefore, have the longest retention time (Rt). It will emerge from the gas chromatograph last.
- A component that adsorbs the least strongly to the stationary phase will spend the least time in the column (will be retained in the column for the shortest time) and will, therefore, have the shortest retention time (Rt). It will emerge from the gas chromatograph first.
- If we consider a 2 component mixture in which component A is more polar than component B then:
- component A will have a longer retention time in a polar column than component B
- component A will have a shorter retention time in a non-polar column than component B
Step 3: Detecting and Recording Results
- The components of the mixture reach the detector at different times due to differences in the time they are retained in the column.
- The component that is retained the shortest time in the column is detected first. The component that is retained the longest time in the column is detected last.
- The detector sends a signal to the chart recorder which results in a peak on the chart paper. The component that is detected first is recorded first. The component that is detected last is recorded last.
Applications
- GC analysis is used to calculate the content of a chemical product, for example in assuring the quality of products in the chemical industry; or measuring toxic substances in soil, air or water.
- Gas chromatography is used in the analysis of:
(a) air-borne pollutants
(b) performance-enhancing drugs in athlete’s urine samples
(c) oil spills
(d) essential oils in perfume preparation
- GC is very accurate if used properly and can measure picomoles of a substance in a 1 ml liquid sample, or parts-per-billion concentrations in gaseous samples.
- Gas Chromatography is used extensively in forensic science. Disciplines as diverse as solid drug dose (pre-consumption form) identification and quantification, arson investigation, paint chip analysis, and toxicology cases, employ GC to identify and quantify various biological specimens and crime-scene evidence.
Advantages
- The use of longer columns and higher velocity of carrier gas permits the fast separation in a matter of a few minutes.
- Higher working temperatures up to 5000C and the possibility of converting any material into a volatile component make gas chromatography one of the most versatile techniques.
- GC is popular for environmental monitoring and industrial applications because it is very reliable and can be run nearly continuously.
- GC is typically used in applications where small, volatile molecules are detected and with non-aqueous solutions.
- GC is favored for non-polar molecules.
Limitations
- Compound to be analyzed should be stable under GC operation conditions.
- They should have a vapor pressure significantly greater than zero.
- Typically, the compounds analyzed are less than 1,000 Da, because it is difficult to vaporize larger compounds.
- The samples are also required to be salt-free; they should not contain ions.
- Very minute amounts of a substance can be measured, but it is often required that the sample must be measured in comparison to a sample containing the pure, suspected substance known as a reference standard.
References
- https://nptel.ac.in/courses/103108100/module7/module7.pdf
- https://en.wikipedia.org/wiki/Gas_chromatography#Carrier_gas_selection_and_flow_rates
- https://www.jove.com/science-education/10187/gas-chromatography-gc-with-flame-ionization-detection
- http://davisson.nat.uni-magdeburg.de/Downloads/Chromatographie.pdf
- https://www.whitman.edu/chemistry/edusolns_software/GC_LC_CE_MS_2017/CH%201%202017.pdf
- http://www.ausetute.com.au/gc.html
- http://web.uniplovdiv.bg/plamenpenchev/mag/books/anchem/Handbook%20of%20Analytical%20Techniques,%202%20Volume%20Set.pdf

THANK U SIR , Students who learn gas chromtography have very clearly remembered that very usefull
Wow wonderful it’s the information am looking for very helpful.
Thanks alot.
This has helped my Work tremendously. And will be looking forward for your assistance, Please.
*My Institution- University of Mkar Benue State Nigeria, procured a New Copy of a Gas Chromatography System and Infrared (IR) System, about 10 years ago nd is not been installed.
Please, can you help me with any of your Technician Specialist that can install the two of them for us?
Thank You
My email is as appeared in my address.
Really helpful
Thanks for sir very most important information
The note was very helpful for my assignment , looking forward for more information on separation analysis techniques.
Thank you.
Thanks for being organised Dr.
Very nicely presented in brief…very easy for a student to understand
Awesome description about GC analysis method…
Really helpfull
Thank you sir, valuable information about GC…
“Higher working temperatures up to 5000C”
this is indeed a high working temperature, but should this be rather 500 °C (still high, though)?
Awesome very organised answer