High-performance liquid chromatography or commonly known as HPLC, is an analytical technique used to separate, identify or quantify each component in a mixture.
The mixture is separated using the basic principle of column chromatography and then identified and quantified by spectroscopy.
In the 1960s, the column chromatography LC with its low-pressure suitable glass columns was further developed to the HPLC with its high-pressure adapted metal columns.
HPLC is thus basically a highly improved form of column liquid chromatography. Instead of a solvent being allowed to drip through a column under gravity, it is forced through under high pressures of up to 400 atmospheres.
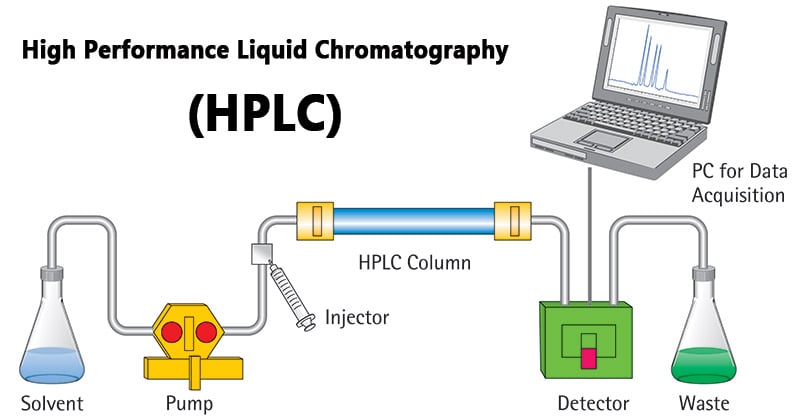
Image Source: Sartorius AG.
Interesting Science Videos
HPLC Principle
- The purification takes place in a separation column between a stationary and a mobile phase.
- The stationary phase is a granular material with very small porous particles in a separation column.
- The mobile phase, on the other hand, is a solvent or solvent mixture which is forced at high pressure through the separation column.
- Via a valve with a connected sample loop, i.e. a small tube or a capillary made of stainless steel, the sample is injected into the mobile phase flow from the pump to the separation column using a syringe.
- Subsequently, the individual components of the sample migrate through the column at different rates because they are retained to a varying degree by interactions with the stationary phase.
- After leaving the column, the individual substances are detected by a suitable detector and passed on as a signal to the HPLC software on the computer.
- At the end of this operation/run, a chromatogram in the HPLC software on the computer is obtained.
- The chromatogram allows the identification and quantification of the different substances.
Instrumentation of HPLC
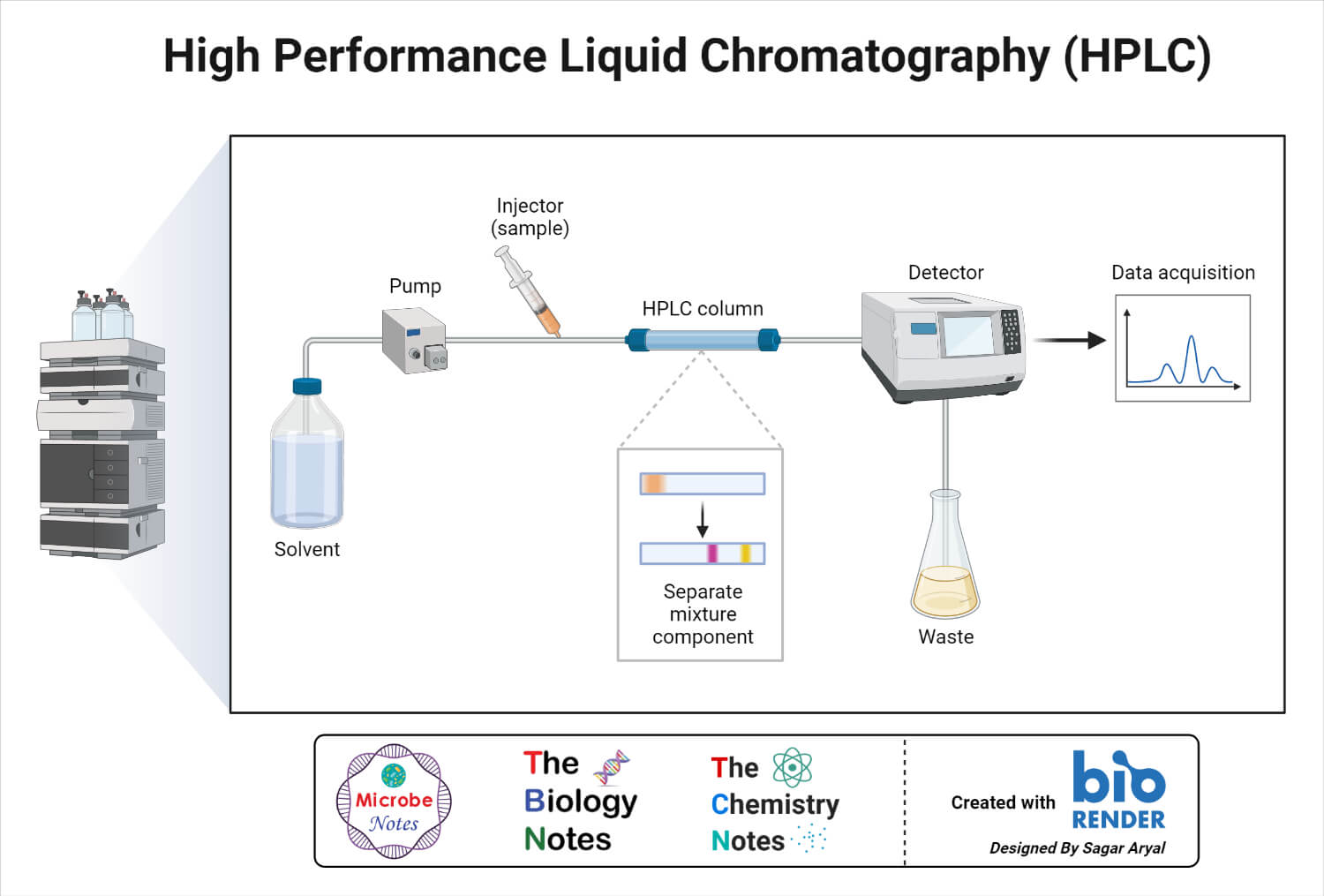
The Pump
- The development of HPLC led to the development of the pump system.
- The pump is positioned in the most upper stream of the liquid chromatography system and generates a flow of eluent from the solvent reservoir into the system.
- High-pressure generation is a “standard” requirement of pumps besides which, it should also to be able to provide a consistent pressure at any condition and a controllable and reproducible flow rate.
- Most pumps used in current LC systems generate the flow by back-and-forth motion of a motor-driven piston (reciprocating pumps). Because of this piston motion, it produces “pulses”.
Injector
- An injector is placed next to the pump.
- The simplest method is to use a syringe, and the sample is introduced to the flow of eluent.
- The most widely used injection method is based on sampling loops.
- The use of the autosampler (auto-injector) system is also widely used that allows repeated injections in a set scheduled-timing.
Column
- The separation is performed inside the column.
- The recent columns are often prepared in a stainless steel housing, instead of glass columns.
- The packing material generally used is silica or polymer gels compared to calcium carbonate.
The eluent used for LC varies from acidic to basic solvents. - Most column housing is made of stainless steel since stainless is tolerant towards a large variety of solvents.
Detector
- Separation of analytes is performed inside the column, whereas a detector is used to observe the obtained separation.
- The composition of the eluent is consistent when no analyte is present. While the presence of analyte changes the composition of the eluent. What detector does is to measure these differences.
- This difference is monitored as a form of an electronic signal. There are different types of detectors available.
Recorder
- The change in eluent detected by a detector is in the form of an electronic signal, and thus it is still not visible to our eyes.
- In older days, the pen (paper)-chart recorder was popularly used. Nowadays, a computer-based data processor (integrator) is more common.
- There are various types of data processors; from a simple system consisting of the in-built printer and word processor while those with software that are specifically designed for an LC system which not only data acquisition but features like peak-fitting, baseline correction, automatic concentration calculation, molecular weight determination, etc.
Degasser
The eluent used for LC analysis may contain gases such as oxygen that are non-visible to our eyes.
- When gas is present in the eluent, this is detected as noise and causes an unstable baseline.
- Degasser uses special polymer membrane tubing to remove gases.
- The numerous very small pores on the surface of the polymer tube allow the air to go through while preventing any liquid to go through the pore.
Column Heater
The LC separation is often largely influenced by the column temperature.
- In order to obtain repeatable results, it is important to keep consistent temperature conditions.
- Also for some analysis, such as sugar and organic acid, better resolutions can be obtained at elevated temperatures (50 to 80°C).
- Thus columns are generally kept inside the column oven (column heater).
Types of HPLC
- Normal phase:
Column packing is polar (e.g silica) and the mobile phase is non-polar. It is used for water-sensitive compounds, geometric isomers, cis-trans isomers, and chiral compounds.
- Reverse phase:
The column packing is non-polar (e.g C18), the mobile phase is water+ miscible solvent (e.g methanol). It can be used for polar, non-polar, ionizable, and ionic samples.
- Ion exchange:
Column packing contains ionic groups and the mobile phase is buffer. It is used to separate anions and cations.
- Size exclusion:
Molecules diffuse into pores of a porous medium and are separated according to their relative size to the pore size. Large molecules elute first and smaller molecules elute later.
Applications of HPLC
The HPLC has developed into a universally applicable method so that it finds its use in almost all areas of chemistry, biochemistry, and pharmacy.
- Analysis of drugs
- Analysis of synthetic polymers
- Analysis of pollutants in environmental analytics
- Determination of drugs in biological matrices
- Isolation of valuable products
- Product purity and quality control of industrial products and fine chemicals
- Separation and purification of biopolymers such as enzymes or nucleic acids
- Water purification
- Pre-concentration of trace components
- Ligand-exchange chromatography
- Ion-exchange chromatography of proteins
- High-pH anion-exchange chromatography of carbohydrates and oligosaccharides
Advantages of HPLC
- Speed
- Efficiency
- Accuracy
- Versatile and extremely precise when it comes to identifying and quantifying chemical components.
Limitations of HPLC
- Cost: Despite its advantages, HPLC can be costly, requiring large quantities of expensive organics.
- Complexity
- HPLC does have low sensitivity for certain compounds, and some cannot be detected as they are irreversibly adsorbed.
- Volatile substances are better separated by gas chromatography.
References
- https://www.shodex.com/en/kouza/a.html
- https://www.alphacrom.com/en/hplc-basics
- https://laboratoryinfo.com/hplc/
- https://sciencing.com/disadvantages-advantages-hplc-5911530.html
- https://www.slideshare.net/krakeshguptha/hplc-26970638
- https://www.chemguide.co.uk/analysis/chromatography/hplc.html
- https://www.azom.com/article.aspx?ArticleID=8468
- https://www.ru.ac.za/media/rhodesuniversity/content/nanotechnology/documents/chromatography%20Augustus.pdf

Thanks 🙏 my assignment is done 😂😂ladies and gentlemen enjoy the weekend
Thank you. It’s helpful
Thank you so much 🙏Sir
The way your written it’s very easy to understand.
Thanks For Information
Wow this is great
excellent
VERY USEFUL INFO
GREAT ITS EASY AND GREAT
Very informative, and clarity.
Perfect identifying of HPLC, Thanks
Very informative and clear explanation to HPLC.
Thanks to explain clearly for HPLC, including the principle, concept ,applications concept and advantages. very effective study material
Perfect information in HPLC
Please add some more basics about detector types, LoQ, LoD etc.
Wow really most educated and intelligent person.thank you sir for one of the best artical
yes its very valuable information
Understood HPLC nonchalantly
Very detailed information about hplc system, it looks very practical and near to reality. I found it one of the best article on HPLC.
thanks for valuable information and good write-up,
Very educative and enlightening. Thanks for your well write up.
Well. Thanks to explain clearly for HPLC, including the principle, applications and advantages. It is very useful.
Perfect material for HPLC..
Its really amazing fact and clearly understable concept, thanks much sir