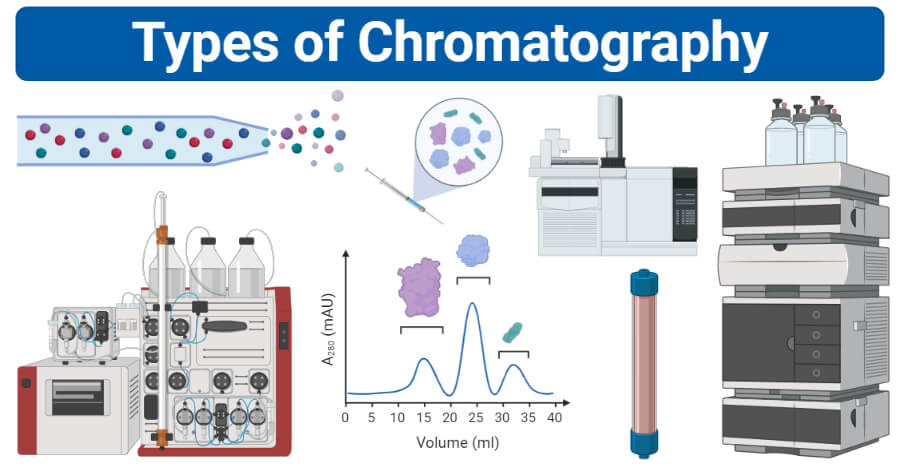
Interesting Science Videos
Chromatography Definition
Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
- A wide range of chromatographic procedures makes use of differences in size, binding affinities, charge, and other properties to separate materials.
- It is a powerful separation tool that is used in all branches of science and is often the only means of separating components from complex mixtures.
- Chromatography is a very useful technique as it allows the separation of components of a mixture on the basis of their nature, structure, size, and other properties.
- Chromatography, in general, is based on the principle that components of a mixture are separated when the mixture added to a mobile phase is moved through a stationary phase (which mostly is a solid surface), resulting in some components of the mixture being attached to the stationary phase. At the same time, the rest is passed along with the mobile phase.
- Thus, there are two essential components of all chromatography techniques.
What is a stationary phase?
The stationary phase in chromatography is the phase that is either a solid or liquid particle attached to a glass or a metal surface on which the components of the mixture to be separated is absorbed selectively.
- The term stationary refers to the fact that this phase remains stationary while the other phase moves.
- Most substances used as stationary phases are porous, thus allowing the attachment of components during chromatography.
- The stationary phase to be selected for a chromatographic process depends on the nature of the components to be separated and the type of chromatography.
- Depending on the type of chromatography gel beads, thin uniform paper, silica, glass, some gases, or even liquid components are used as a stationary phase.
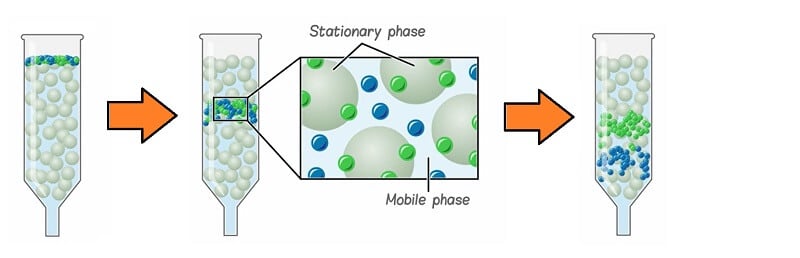
Image Source: Diseñada por Cerotec Estudios.
What is the mobile phase?
The mobile phase in chromatography is the phase that is either liquid or gas that is passed through a chromatographic system where the components of the mixture are separated at different raters by adsorbing them to the stationary phase.
- The mobile phase is the solvent that carries the mixture as it moves down the stationary phase.
- The term mobile indicates that the phase is moving down the chromatographic system, whereas the other phase remains stationary.
- Substances used as mobile phases are selected for a chromatographic process depending on the nature of the components to be separated and the type of chromatography.
- Alcohol, water, acetic acid, acetone, or some gases are the commonly used mobile phase in different chromatographic techniques.
Types of Chromatography
1. Affinity chromatography
Affinity chromatography is a separation technique where the components of a mixture are separated based on their affinity towards the stationary phase of the system.
Principle of Affinity chromatography
- This chromatography technique is based on the principle that components of a mixture are separated when the element having an affinity towards the stationary phase binds to the stationary phase. In contrast, other components are eluted with the mobile phase.
- The substrate/ ligand is bound to the stationary phase so that the reactive sites for the binding of components are exposed.
- Now, the mixture is passed through the mobile phase where the components with binding sites for the substrate bind to the substrate on the stationary phase while the rest of the components are eluted out with the mobile phase.
- The components attached to the stationary phase are then eluted by changing the pH, ionic strength, or other conditions.
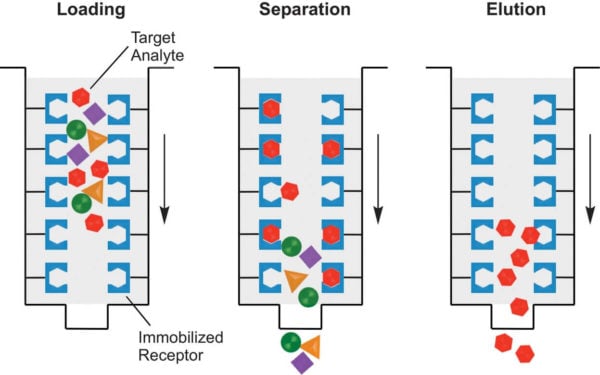
Figure: Affinity chromatography. Image Source: Creative Biostructure.
Steps of Affinity chromatography
- The column is prepared by loading it with solid support like agarose or cellulose, onto which the substrate/ ligand with the spacer arm, is attached.
- The mobile phase containing the mixture is poured into the column at a constant rate.
- Once the process is complete, the ligand-molecule complex is eluted from the stationary phase by changing the conditions that favor the separation of ligand and components of the mixture.
Uses of Affinity chromatography
- Affinity chromatography is used as a staple separation technique from enzymes and other proteins.
- This principle is also applied in the in vitro antigen-antibody reactions.
- This technique is used for the separation of components as well as the removal of impurities from a mixture.
- Affinity chromatography can be used in the detection of mutation and nucleotide polymorphisms in nucleic acids.
Examples of Affinity chromatography
- The purification of coli β-galactosidase from a mixture of proteins using the p-aminophenyl-1-thio-β-D-galactopyranosyl agarose as the affinity matrix.
- The removal of excess albumin and α2-macroglobulin from the serum albumin.
2. Anion exchange chromatography
Anion exchange chromatography is the separation technique for negatively charged molecules by their interaction with the positively charged stationary phase in the form of ion-exchange resin.
Principle of Anion exchange chromatography
- This technique is based on the principle of attraction of positively charged resin and the negatively charged analyte. Here the exchange of positively charged ions takes place to remove the negatively charged molecules.
- The stationary phase is first coated with positive charges where the components of the mixture with negative charges will bind.
- An anion exchange resin with a higher affinity to the negatively charged components then binds the components, displacing the positively charged resin.
- The anion exchange resin-component complex then is removed by using different buffers.
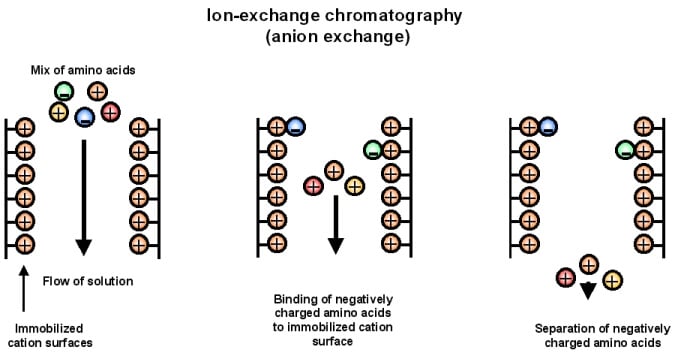
Figure: Anion exchange chromatography. Image Source: https://sites.google.com/site/chromospectrum/i-exchange
Steps of Anion exchange chromatography
- A column packed with positively charged resin is taken as the stationary phase.
- The mixture with the charged particles is then passed down the column where the negatively charged molecules bind to the positively charged resins.
- The anion exchange resin is then passed through the column where the negatively charged molecules now bind to the anion exchange resin displacing the positively charged resin.
- Now an appropriate buffer is applied to the column to separate the complex of anion exchange resins and the charged molecules.
Uses of Anion exchange chromatography
- Anion exchange chromatography is used to separate proteins and amino acids from their mixtures.
- Negatively charged nucleic acids can be separated, which helps in further analysis of the nucleic acids.
- This method can also be used for water purification where the anions are exchanged for hydroxyl ions.
- Anion exchange resins can be used for the separation of metals as they usually have negatively charged complexes that are bound to the anion exchangers.
Examples of Anion exchange chromatography
- The separation of nucleic acids from a mixture obtained after cell destruction.
- The separation of proteins from the crude mixture obtained from the blood serum.
3. Cation exchange chromatography
Anion exchange chromatography is the separation technique for positively charged molecules by their interaction with negatively charged stationary phase in the form of ion-exchange resin.
Principle of Cation exchange chromatography
- This technique is based on the principle of attraction of negatively charged resin and the positively charged analyte. Here the exchange of negatively charged ions takes place to remove the positively charged molecules.
- The stationary phase is first coated with negative charges where the components of the mixture with positive charges will bind.
- A cation exchange resin with a higher affinity to the positively charged components then binds the components, displacing the negatively charged resin.
- The cation exchange resin-component complex then is removed by using different buffers.
Steps of Cation exchange chromatography
- A column packed with negatively charged resin is taken as the stationary phase.
- The mixture with the charged particles is then passed down the column where the positively charged molecules bind to the negatively charged resins.
- The cation exchange resin is then passed through the column where the positively charged molecules now bind to the cation exchange resin displacing the negatively charged resin.
- Now an appropriate buffer is applied to the column to separate the complex of cation exchange resins and the charged molecules.
Uses of Cation exchange chromatography
- Cation exchange chromatography is used for the analysis of the products obtained after the hydrolysis of nucleic acids.
- This can also be used for the separation of metals where the metal ions themselves bind to the negatively charged resins to remove the negatively charged complexes.
- Cation exchange chromatography helps in purification of water by exchanging the positively charged ion by the hydrogen ions.
- It is also used to analyze the rocks and other inorganic molecules.
Examples of Cation exchange chromatography
- The separation of positively charged lanthanoid ions obtained from the earth’s crust.
- The determination of total dissolved salts in natural waters by analyzing the presence of calcium ions.
4. Column chromatography
Column chromatography is the separation technique where the components in a mixture are separated on the basis of their differential adsorption with the stationary phase, resulting in them moving at different speeds when passed through a column.
It is a solid-liquid chromatography technique in which the stationary phase is a solid & mobile phase is a liquid or gas.
Principle of Column chromatography
- This technique is based on the principle of differential adsorption where different molecules in a mixture have different affinities with the absorbent present on the stationary phase.
- The molecules having higher affinity remain adsorbed for a longer time decreasing their speed of movement through the column.
- However, the molecules with lower affinity move with a faster movement, thus allowing the molecules to be separated in different fractions.
- Here, the stationary phase in the column chromatography also termed the absorbent, is a solid (mostly silica) and the mobile phase is a liquid that allows the molecules to move through the column smoothly.
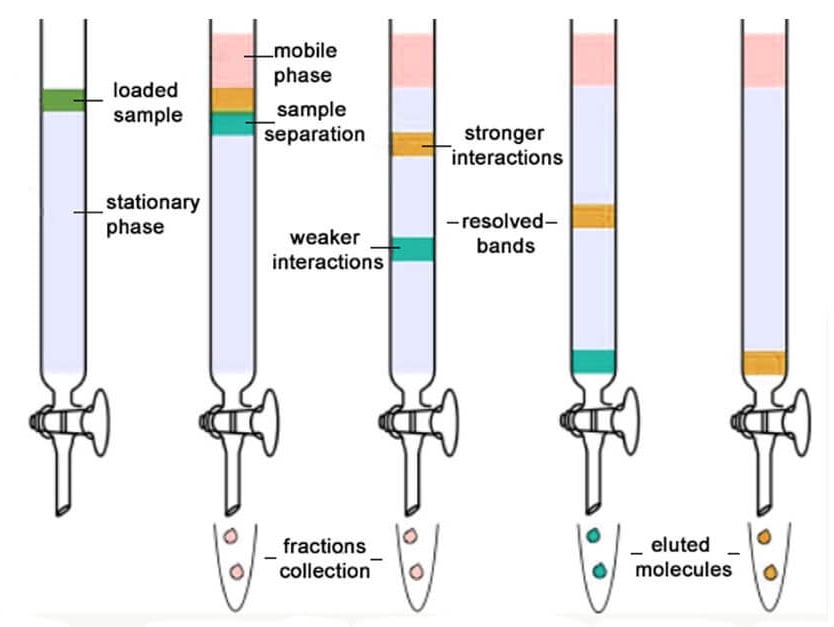
Figure: Column chromatography. Image Source: PrepGenie.
Steps of Column chromatography
- The column is prepared by taking a glass tube that is dried and coated with a thin, uniform layer of stationary phase (cellulose, silica).
- Then the sample is prepared by adding the mixture to the mobile phase. The sample is introduced into the column from the top and is allowed to pass the sample under the influence of gravity.
- The molecules bound to the column are separated by elution technique where either solution of the same polarity is used (isocratic technique), or different samples with different polarities are used (gradient technique).
- The separated molecules can further be analyzed for various purposes.
Uses of Column chromatography
- Column chromatography is routinely used for the separation of impurities and purification of various biological mixtures.
- This technique can also be used for the isolation of active molecules and metabolites from various samples.
- Column chromatography is increasingly used for the detection of drugs in crude extracts.
Examples of Column chromatography
- Extraction of pesticides from solid food samples of animal origin containing lipids, waxes, and pigments.
- Synthesis of Pramlintide which is an analog of Amylin, a peptide hormone, for treating type 1 and type 2 Diabetics.
- Purification of bioactive glycolipids, showing antiviral activity towards HSV-1 (Herpes Virus).
5. Flash chromatography
Flash chromatography is a separation technique where smaller sizes of gel particles are used as stationary phase, and pressurized gas is used to drive the solvent through the column.
Principle of Flash chromatography
- The principle of flash chromatography is similar to that of column chromatography, where the components are separated on the basis of their differential adsorption to the stationary phase.
- The sample applied is passed by using a pressurized gas that makes the process faster and more efficient.
- Molecules bind to the stationary phase on the basis of their affinity while the rest of the solvent is eluted out by applying the pressured gas which quickens the process.
- Here, the stationary phase is solid, the mobile phase and the elution solution are liquid, and an additional pressurized gas is used.
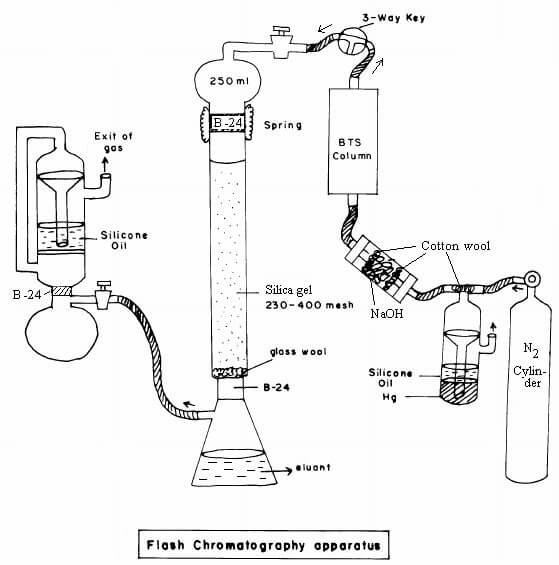
Figure: Flash chromatography. Image Source: Siddhartha S. Baisya (Research Gate)
Steps of Flash chromatography
- The column is prepared by taking a glass tube that is dried and coated with a thin, uniform layer of stationary phase (cellulose, silica). The bottom and top of the column are packed with cotton wool to prevent the gel from escaping.
- Then the sample is prepared by adding the mixture to the mobile phase. The sample is introduced into the column from the top, and a pumped sample is used to pass the sample at a constant rate.
- The molecules bound to the column are separated by elution solution where either solution of the same polarity is used (isocratic technique), or different samples with different polarities are used (gradient technique).
- The elution solvent is applied with a constant minimum pressure required to move the solute down the column.
- The separated molecules can further be analyzed for various purposes.
Uses of Flash chromatography
- Flash chromatography is used as a rapid and more efficient method of separation of components of different mixtures.
- It is used for the removal of impurities from crude extracts of natural and synthetic mixtures.
6. Gas chromatography
Gas chromatography is a separation technique in which the molecules are separated on the basis of their retention time depending on the affinity of the molecules to the stationary phase.
The sample is either liquid or gas that is vaporized in the injection point.
Principle of Gas chromatography
- Gas chromatography is based on the principle that components having a higher affinity to the stationary phase have a higher retention time as they take a longer time to come out of the column.
- However, the components having a higher affinity to the stationary phase have less retention time as they move along with the mobile phase.
- The mobile phase is a gas, mostly helium, that carries the sample through the column.
- The sample once injected in converted into the vapor stage is then passed through a detector to determine the retention time.
- The components are collected separately as they come out of the stationary phase at different times.
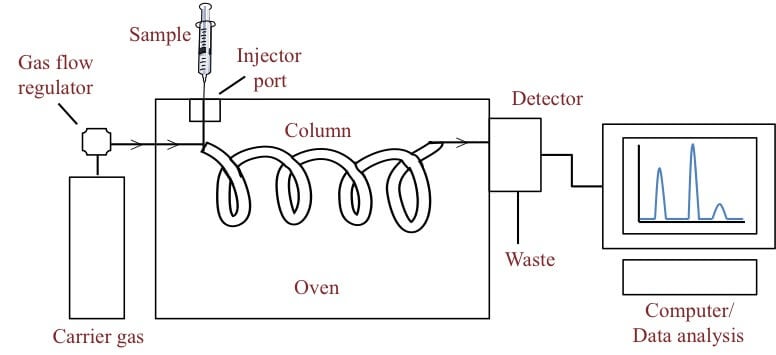
Figure: Gas chromatography. Image Source: Bitesize Bio.
Steps of Gas chromatography
- The sample is injected into the column where it is vaporized into a gaseous state. The vapourised component than mixes with the mobile phase to be carried through the rest of the column.
- The column is set with the stationary phase where the molecules are separated on the basis of their affinity to the stationary phase.
- The components of the mixture reach the detector at different times due to differences in the time they are retained in the column.
Uses of Gas chromatography
- This technique is used to calculate the concentration of different chemicals in various samples.
- This is used in the analysis of air pollutants, oil spills, and other samples.
- Gas chromatography can also be used in forensic science to identify and quantify various biological samples found in the crime scene.
Examples of Gas chromatography
- The identification of performance-inducing drug in the athlete’s urine.
- The separation and quantification of a solid drug in soil and water samples.
7. Gel filtration chromatography/ Gel permeation chromatography/ Size exclusion chromatography/ Molecular sieve chromatography
Gel-filtration chromatography is a form of partition chromatography used to separate molecules of different molecular sizes.
This technique has also frequently been referred to by various other names, including gel-permeation, gel-exclusion, size- exclusion, and molecular- sieve chromatography.
Principle
- Molecules are partitioned between a mobile phase and a stationary phase as a function of their relative sizes.
- The stationary phase is a matrix of porous polymer which have pores of specific sizes.
- When the sample is injected with the mobile phase, the mobile phase occupies the pores of the stationary phase.
- If the size of the molecules is appropriate enough to enter the pores, they remain in the pores partly or wholly.
- However, molecules with a larger size are retained from entering the pores, causing them to be moved with the mobile phase, out of the column.
- If the mobile phase used in an aqueous solution, the process is termed gel filtration chromatography.
- If the mobile phase used is an organic solvent, it is termed as gel permeation chromatography.
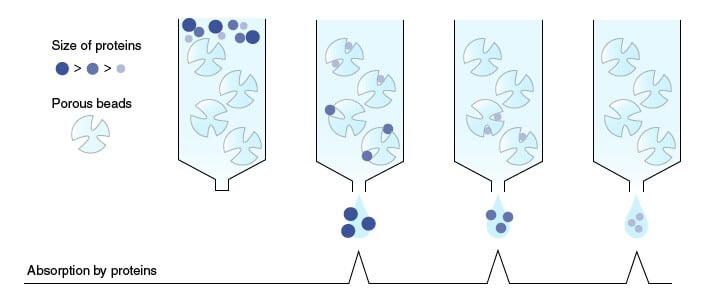
Figure: Gel-filtration chromatography. Image Source: MBL Life Science.
Steps
- The column is filled with semi-permeable, porous polymer gel beads with a well-defined range of pore sizes.
- The sample, mixed with the mobile phase, is then injected into the column from the top of the column.
- The molecules bound to the column are separated by elution solution where either solution of the same polarity is used (isocratic technique), or different samples with different polarities are used (gradient technique).
- Elution conditions (pH, essential ions, cofactors, protease inhibitors, etc.) can be selected, which will complement the requirements of the molecule of interest.
Uses
- One of the principal advantages of gel-filtration chromatography is that separation can be performed under conditions specifically designed to maintain the stability and activity of the molecule of interest without compromising resolution.
- The absence of a molecule-matrix binding step also prevents unnecessary damage to fragile molecules, ensuring that gel-filtration separations generally give high recoveries of activity.
- Because of its unique mode of separation, gel-filtration chromatography has been used successfully in the purification of proteins and peptides from various sources.
- Gel-filtration chromatography has been used to separate various nucleic acid species such as DNA, RNA, and tRNA as well as their constituent bases, adenine, guanine, thymine, cytosine, and uracil.
Examples
- The separation of recombinant human granulocyte colony-stimulating factor (rhG-CSF) from inclusion bodies in high yield by urea-gradient size-exclusion chromatography.
- The separation of hen egg lysozyme using both acrylamide- and dextran-based gel columns.
8. High-performance liquid chromatography (HPLC)
High-performance liquid chromatography is a modified form of column chromatography where the components of a mixture are separated on the basis of their affinity with the stationary phase.
Principle of HPLC
- This technique is based on the principle of differential adsorption where different molecules in a mixture have a varying degree of interactions with the absorbent present on the stationary phase.
- The molecules having higher affinity remain adsorbed for a longer time decreasing their speed of movement through the column.
- However, the molecules with lower affinity move with a faster movement, thus allowing the molecules to be separated in different fractions.
- This process is slightly different from the column chromatography as in this case; the solvent is forced under high pressures of up to 400 atmospheres instead of allowing it to drip down under gravity.
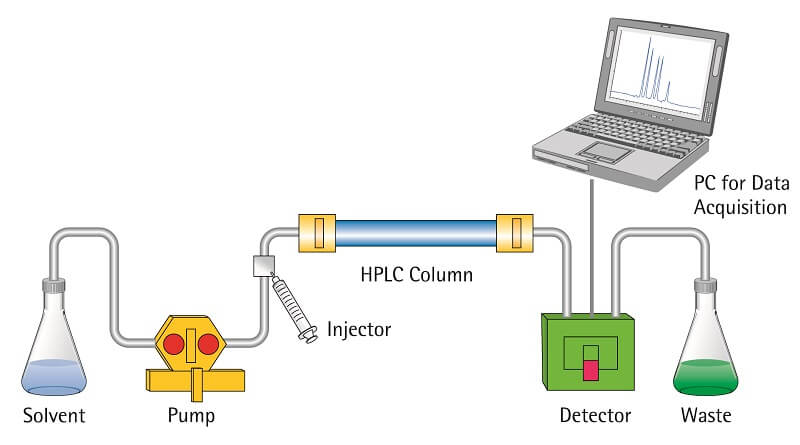
Figure: High-performance liquid chromatography (HPLC). Image Source: Toppr.
Steps of HPLC
- The column is prepared by taking a glass tube that is dried and coated with a thin, uniform layer of stationary phase (cellulose, silica).
- Then the sample is prepared by adding the mixture to the mobile phase. The sample is introduced into the column from the top, and a high-pressure pump is used to pass the sample at a constant rate.
- The mobile phase then moves down to a detector that detects molecules at a certain absorbance wavelength.
- The separated molecules can further be analyzed for various purposes.
Uses of HPLC
- High-performance liquid chromatography is used in the analysis of pollutants present in environmental samples.
- It is performed to maintain product purity and quality control of various industrial productions.
- This technique can also be used to separate different biological molecules like proteins and nucleic acids.
- The increased speed of this technique makes the process faster and more effective.
Example of HPLC
- High-performance liquid chromatography has been performed to test the efficiency of different antibodies against diseases like Ebola.
9. Hydrophobic interaction chromatography
Hydrophobic interaction chromatography is the separation technique that separates molecules on the basis of their degree of hydrophobicity.
Principle of Hydrophobic interaction chromatography
- The principle of hydrophobic interaction chromatography is based on the interaction between two molecules with hydrophobic groups.
- Here, the stationary phase is solid support applied with both hydrophobic and hydrophilic groups.
- The solvent molecules containing hydrophobic regions interact with the hydrophobic groups, thus separating them from the molecules with hydrophilic groups.
- The interaction is then reversed by applying an elution solution with decreasing salt gradient, which causes the molecules with hydrophobic groups to be separated from the stationary phase.
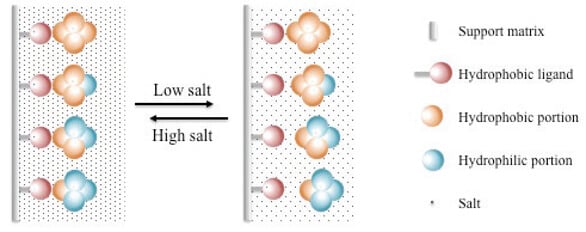
Figure: Hydrophobic interaction chromatography. Image Source: American Pharmaceutical Review.
Steps of Hydrophobic interaction chromatography
- The column is prepared with a glass tube applied with solid support like silica gel, upon which hydrophobic groups like phenyl, octyl butyl, are attached.
- The sample is prepared by adding the mixture to the mobile phase.
- The sample is then injected into the column from the top of the column.
- The molecules with hydrophobic groups form an interaction with the hydrophobic groups of the stationary phase. In contrast, the molecules without such groups move out of the column with the mobile phase.
- Then a particular elution solution with decreasing salt gradient is then passed into the column that removes the bound molecules from the stationary phase.
Uses of Hydrophobic interaction chromatography
- Hydrophobic interaction chromatography is extremely important for the separation of proteins with hydrophobic groups.
- This technique is more appropriate than other methods, as this technique results in minimum denaturation activities.
- Similarly, this method can also be applied to the separation of other organic compounds with hydrophobic groups.
- This allows the separation of hydrophilic and hydrophobic biological molecules from each other.
Example of Hydrophobic interaction chromatography
- The separation of plant proteins from the crude extracts.
10. Ion exchange chromatography
Ion exchange chromatography is the separation technique for charged molecules by their interaction with the oppositely charged stationary phase in the form of ion-exchange resin.
Principle of Ion exchange chromatography
- This technique is based on the principle of attraction of charged resin and the oppositely charged analyte. Here the exchange of negatively/ positively charged ions takes place to remove the charged molecules.
- The stationary phase is first coated with particular charges where the components of the mixture with opposite charges will bind.
- A cation or anion exchange resin with a higher affinity to the charged components then binds the components, displacing the oppositely charged resin.
- The cation or anion exchange resin-component complex then is removed by using different buffers.
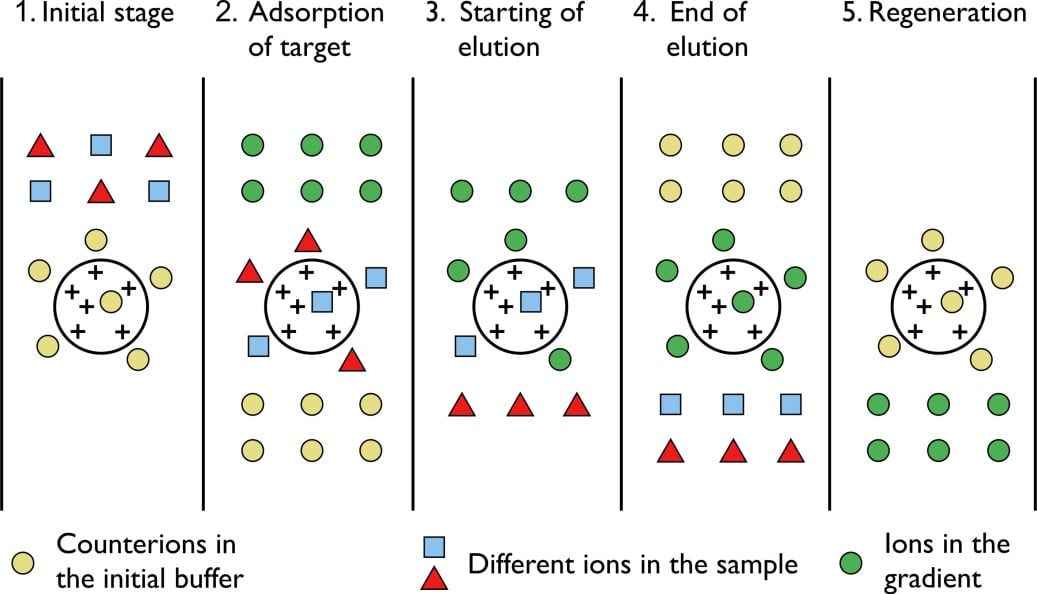
Figure: Ion exchange chromatography.
Steps of Ion exchange chromatography
- A column packed with charged resin that can either be positively charged or negatively charged is taken as the stationary phase.
- The mixture with the charged particles is then passed down the column where the charged molecules bind to the oppositely charged resins.
- If a cation exchange resin is used, the positively charged molecules now bind to the cation exchange resin displacing the negatively charged resin.
- Similarly, if an anion exchange resin is used, the negatively charged molecules bind to the anion exchange resin displacing the positively charged resin.
- Now an appropriate buffer is applied to the column to separate the complex of charged exchange resins and the charged molecules.
Uses of Ion exchange chromatography
- Ion exchange chromatography is used in the purification of water where the positively charged ions are replaced by hydrogen ions, and the negatively charged ions are replaced by hydroxyl ions.
- This method also works as an effective method for the analysis of the products formed after hydrolysis of nucleic acids.
- The separation of metals and other inorganic compounds is also facilitated by the ion-exchange chromatography.
Examples of Ion exchange chromatography
- The separation of positively charged lanthanoid ions obtained from the earth’s crust.
- The separation of proteins from the crude mixture obtained from the blood serum.
11. Liquid chromatography
Liquid chromatography is a separation technique where the mobile phase used is liquid, and the separation can take place either in a column or a plain surface.
Principle of Liquid chromatography
- The process of liquid chromatography is based on the principle for the affinity of the molecules to the mobile phase.
- If the components to be separated have a higher affinity to the mobile phase, the molecules move along with the mobile phase and come out of the column faster.
- However, if the components have a lower degree of interaction with the mobile phase, the molecules move slowly and thus come out of the column later.
- Thus, if two molecules in a mixture have different polarities and the mobile phase is of a distinct polarity, the two molecules will move at different speeds through the stationary phase.
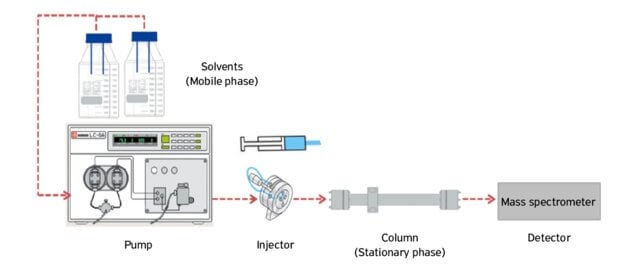
Figure: Liquid chromatography. Image Source: Vânia Margaret Flosi Paschoalin (Researchgate).
Steps of Liquid chromatography
- The column or paper is prepared where the stationary phase (cellulose or silica) is applied on the solid support.
- The sample is added to the liquid mobile phase, which is then injected into the chromatographic system.
- The mobile phase moves through the stationary phase before coming out of the column or the edge of the paper.
- An elution solution is applied to the system to separate the molecules from the stationary phase.
Uses of Liquid chromatography
- Liquid chromatography is an effective method for the separation of a colored solution as they form two separate bands after separation.
- This method can also be used over other techniques as it is quite simple and less expensive.
- It can be used for the separation of solid molecules that are insoluble in water.
Examples of Liquid chromatography
- High-performance liquid chromatography is a modified form of liquid chromatography that is used in the research regarding biological molecules.
12. Paper chromatography
Paper chromatography is a separation technique where the separation is performed on a specialized paper.
Principle of Paper chromatography
- Paper chromatography is of two types based on two different principles.
- The first is the paper adsorption chromatography that is based on the varying degree of interaction between the molecules and the stationary phase.
- The molecules having higher affinity remain adsorbed for a longer time decreasing their speed of movement through the column.
- However, the molecules with lower affinity move with a faster movement, thus allowing the molecules to be separated in different fractions.
- The second type of paper chromatography is the paper partition chromatography. It is based on the principle that the moisture on the cellulose paper acts as a stationary phase for the molecules moving with the mobile phase.
- The separation of the molecules is thus based on how strongly they adsorb onto the stationary phase.
- An additional concept of ‘retention factor’ is applied during the separation of molecules in the paper chromatography.
- The retention value for a molecule is determined as a ratio of distance traveled by the molecule to the distance traveled by the mobile phase.
- The retention value of different molecules can be used to differentiate those molecules.
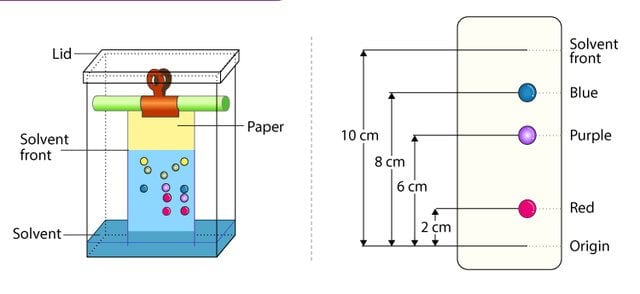
Figure: Paper chromatography. Image Source: Enyoh Christian Ebere (Researchgate).
Steps of Paper chromatography
- The stationary phase is selected as a fine quality cellulosic paper.
- Different combinations of organic and inorganic solvents are taken as the mobile phase.
- About 2-200 µl of the sample solution is injected at the baseline of the paper, and it is allowed to air dry.
- The sample loaded paper is then carefully dipped into the mobile phase not more than the height of 1 cm.
- After the mobile phase reaches near the edge of the paper, the paper is taken out.
- The retention factor is calculated, and the separated components are detected by different techniques.
Uses of Paper chromatography
- Paper chromatography is performed to detect the purity of various pharmaceutical products.
- It can also be employed to detect contamination in various samples, like food and beverages.
- This method can also be used for the separation of impurities from various industrial products.
- The analysis of the reaction mixtures in chemical labs is also conducted via paper chromatography.
Examples of Paper chromatography
- Paper chromatography is used in the separation of mixtures of inks or other colored drinks.
13. Reverse-phase chromatography
Reverse-phase chromatography is a liquid chromatography technique where the separation of molecules is achieved through hydrophobic interaction between the liquid mobile phase and the stationary phase.
Principle of Reverse-phase chromatography
- The principle of reverse phase chromatography is based on the interaction between two molecules with hydrophobic groups.
- Here, the stationary phase is solid support applied with both hydrophobic and hydrophilic groups.
- The solvent molecules containing hydrophobic regions interact with the hydrophobic groups, thus separating them from the molecules with hydrophilic groups.
- The interaction is then reversed by applying an elution solution with decreasing salt gradient, which causes the molecules with hydrophobic groups to be separated from the stationary phase.
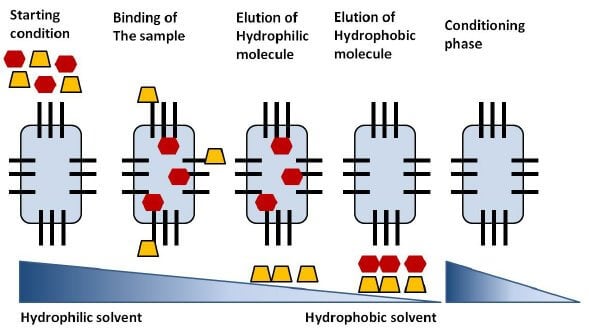
Figure: Steps of a reversed-phase chromatography separation. Image Source: Annette C Moser (Researchgate).
Steps of Reverse-phase chromatography
- The column is prepared with a glass tube applied with solid support like silica gel, upon which hydrophobic groups like phenyl, octyl butyl, are attached.
- The sample is prepared by adding the mixture to the mobile phase of organic and inorganic solvents.
- The sample is then injected into the column from the top of the column.
- The molecules with hydrophobic groups form an interaction with the hydrophobic groups of the stationary phase. In contrast, the molecules without such groups move out of the column with the mobile phase.
- Then a particular elution solution with decreasing salt gradient is then passed into the column that removes the bound molecules from the stationary phase.
Uses of Reverse-phase chromatography
- Reverse chromatography, in combination with high-performance liquid chromatography, is increasingly used for the separation of biomolecules.
- This is also used in the study of the analysis of drugs, metabolites, and active molecules.
- It can also be used to remove impurities from various environmental samples.
Examples of Reverse-phase chromatography
- Hydrophobic interaction chromatography is an example of reverse phase chromatography where this technique is used to separate proteins from their mixtures.
14. Thin-layer chromatography (TLC)
Thin-layer chromatography is a separation technique where the stationary phase is applied as a thin layer on a solid support plate with a liquid mobile phase.
Principle of Thin-layer chromatography (TLC)
- This chromatography technique is based on the principle that components of a mixture are separated when the component having an affinity towards the stationary phase binds to the stationary phase. In contrast, other components are eluted with the mobile phase.
- The substrate/ ligand is bound to the stationary phase so that the reactive sites for the binding of components are exposed.
- Now, the mixture is passed through the mobile phase where the components with binding sites for the substrate bind to the substrate on the stationary phase while the rest of the components are eluted out with the mobile phase.
- After separation, the molecules are seen as spots at a different location throughout the stationary phase.
- The detection of molecules is performed by various techniques.
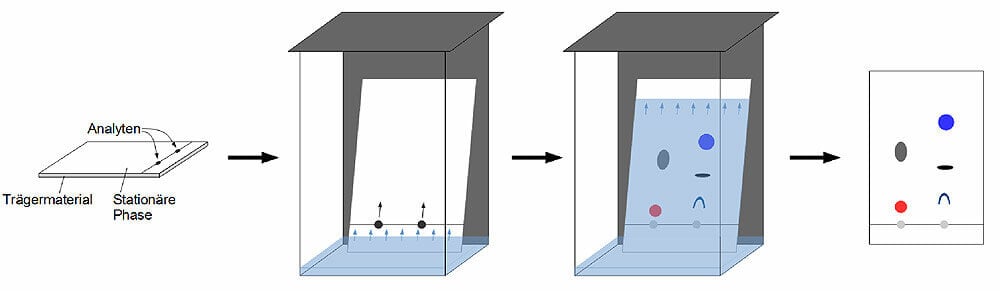
Figure: Thin-layer chromatography (TLC). Image Source: MZ-Analysentechnik GmbH.
Steps of Thin-layer chromatography (TLC)
- The stationary phase is uniformly applied on the solid support (glass, thin plate or aluminum foil) and dried.
- The sample is injected as spots on the stationary phase about 1 cm above the edge of the plate.
- The sample loaded plate is then carefully dipped into the mobile phase not more than the height of 1 cm.
- After the mobile phase reaches near the edge of the plate, the plate is taken out.
- The retention factor is calculated as in paper chromatography, and the separated components are detected by different techniques.
Uses of Thin-layer chromatography (TLC)
- Thin-layer chromatography is routinely performed in laboratories to identify different substances present in a mixture.
- This technique helps in the analysis of fibers in forensics.
- TLC also allows the assay of various pharmaceutical products.
- It aids in the identification of medicinal plants and their composition.
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- Ó’Fágáin, C., Cummins, P. M., & O’Connor, B. F. (2017). Gel-Filtration Chromatography. Methods in molecular biology (Clifton, N.J.), 1485, 15–25. https://doi.org/10.1007/978-1-4939-6412-3_2
Sources
- 3% – https://rd.springer.com/protocol/10.1007/978-1-4939-6412-3_2
- 1% – https://www.toppr.com/ask/question/chromatography-is-a-method-of-separation-which-works-on-the-principle-of/
- 1% – https://www.researchgate.net/publication/47556773_Hydrophobic_Interaction_Chromatography
- 1% – https://brainly.in/question/17535676
- 1% – https://answersdrive.com/what-is-the-stationary-phase-in-chromatography-73174
- <1% – https://www.workplacetesting.com/definition/1293/mobile-phase
- <1% – https://www.ukessays.com/essays/biology/the-separation-of-compounds-of-different-polarity-biology-essay.php
- <1% – https://www.thoughtco.com/gas-chromatography-4138098
- <1% – https://www.studyread.com/types-of-chromatography/
- <1% – https://www.studyread.com/chromatography-definition-principle-techniques/
- <1% – https://www.slideshare.net/shishirkawde/ion-exchange-chromatography
- <1% – https://www.slideshare.net/jabirrahaman/mobile-phase-in-chromatography
- <1% – https://www.slideshare.net/GamalAbdulHamid/high-performance-liquid-chromatograph-hplc
- <1% – https://www.slideshare.net/ajithnandanam/hydrophobic-interaction-chromatography-hic-theory-and-principle
- <1% – https://www.shimadzu.com/an/gc/support/fundamentals/gc.html
- <1% – https://www.researchgate.net/publication/309743873_Separation_techniques_Chromatography
- <1% – https://www.researchgate.net/publication/259701045_Modeling_of_salt_and_pH_gradient_elution_in_ion-exchange_chromatography
- <1% – https://www.researchgate.net/publication/223628077_Separation_and_removal_of_metal_ions_from_dilute_solutions_using_micellar-enhanced_ultrafiltration
- <1% – https://www.quora.com/What-is-the-basic-principle-of-high-performance-liquid-chromatography-HPLC
- <1% – https://www.quora.com/What-is-Adsorption-Chromatography
- <1% – https://www.phmethods.net/sites/default/files/10.5530phm.2017.8.1.pdf
- <1% – https://www.pharmatutor.org/articles/flash-chromatography-area-applications
- <1% – https://www.ncbi.nlm.nih.gov/pubmed/6175345
- <1% – https://www.ncbi.nlm.nih.gov/pubmed/18179225
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3174051/
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1529816/
- <1% – https://www.mdpi.com/2223-7747/6/4/42/htm
- <1% – https://www.lenntech.com/Data-sheets/Ion-Exchange-for-Dummies-RH.pdf
- <1% – https://www.lenntech.com/Data-sheets/Dowex-Ion-Exchange-Resins-Fundamentals-L.pdf
- <1% – https://www.jove.com/science-education/10187/gas-chromatography-gc-with-flame-ionization-detection
- <1% – https://www.jove.com/science-education/10156/high-performance-liquid-chromatography-hplc
- <1% – https://www.cytivalifesciences.com/en/us/solutions/protein-research/knowledge-center/protein-purification-methods/ion-exchange-chromatography
- <1% – https://www.coursehero.com/file/p745k85/Smaller-molecules-enter-the-pores-of-the-resin-and-diffuse-further-into-the/
- <1% – https://www.coursehero.com/file/p2nmhim/Figure-1-shows-a-beaker-containing-mobile-phase-and-a-prepared-paper-stationary/
- <1% – https://www.column-chromatography.com/blogs/application-of-column-chromatography-in-pharmacy
- <1% – https://www.chemguide.co.uk/analysis/chromatography/paper.html
- <1% – https://www.chemguide.co.uk/analysis/chromatography/hplc.html
- <1% – https://www.britannica.com/science/stationary-phase-chromatography
- <1% – https://www.britannica.com/science/paper-chromatography
- <1% – https://www.britannica.com/science/cation-exchange-resin
- <1% – https://www.bio-rad.com/en-us/applications-technologies/cation-exchange-chromatography?ID=MWHB018UU
- <1% – https://www.bio-rad.com/en-us/applications-technologies/anion-exchange-chromatography?ID=MWHAZ4C4S
- <1% – https://www.bio-rad.com/en-uk/applications-technologies/anion-exchange-chromatography?ID=MWHAZ4C4S
- <1% – https://www.bio-rad.com/de-de/applications-technologies/cation-exchange-chromatography?ID=MWHB018UU
- <1% – https://www.biologydiscussion.com/biochemistry/chromatography-techniques/top-12-types-of-chromatographic-techniques-biochemistry/12730
- <1% – https://www.bbc.co.uk/bitesize/guides/zgt6b82/revision/3
- <1% – https://www.bbc.co.uk/bitesize/guides/zgbqtfr/revision/7
- <1% – https://www.answers.com/Q/What_is_the_role_of_stationary_phase_in_chromatography
- <1% – https://vlab.amrita.edu/?sub=2&brch=191&sim=341&cnt=1
- <1% – https://satyapsingh.files.wordpress.com/2012/09/chromatography-and-distillation.pdf
- <1% – https://sargenttexas.org/chromatography-2/
- <1% – https://pediaa.com/what-is-the-difference-between-mobile-phase-and-stationary-phase/
- <1% – https://pediaa.com/difference-between-normal-phase-and-reverse-phase-chromatography/
- <1% – https://oneofchemistry.blogspot.com/2011/10/thin-layer-chromatography-and-column.html
- <1% – https://medical-dictionary.thefreedictionary.com/Flash+column+chromatography
- <1% – https://link.springer.com/chapter/10.1007%2F978-3-319-45776-5_12
- <1% – https://instrumentationtools.com/chromatography-questions-answers/
- <1% – https://core.ac.uk/download/pdf/147603501.pdf
- <1% – https://chrominfo.blogspot.com/2020/06/
- <1% – https://chem-net.blogspot.com/2013/07/what-is-gas-chromatography-gc.html
- <1% – https://chemistry.missouri.edu/sites/default/files/class-files/new_chromatography_lab_2.pdf
- <1% – https://chem.libretexts.org/Bookshelves/Ancillary_Materials/Demos%2C_Techniques%2C_and_Experiments/General_Lab_Techniques/Thin_Layer_Chromatography
- <1% – https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumental_Analysis/Chromatography/High_Performance_Liquid_Chromatography
- <1% – https://byjus.com/chemistry/thin-layer-chromatography/
- <1% – https://answersdrive.com/what-role-does-polarity-play-in-chromatography-7022293
- <1% – http://www.open.edu/openlearn/science-maths-technology/science/biology/nucleic-acids-and-chromatin/content-section-2.4
- <1% – http://vlab.amrita.edu/?sub=2&brch=191&sim=341&cnt=1
- <1% – http://orgchemboulder.com/Technique/Procedures/Columnchrom/Columnchrom.shtml
- <1% – http://europepmc.org/abstract/MED/28058406
- <1% – http://biotechisfuture.weebly.com/uploads/1/4/1/6/14160671/affinity_chromatography_1.pdf

well explained
Excellent work ….thanks to microbenote
Complete chromatography nicely explained 👍👍