Chromatography is an important biophysical technique that enables the separation, identification, and purification of the components of a mixture for qualitative and quantitative analysis.
Chromatography is a separation technique in which a mobile phase carrying a mixture is caused to move in contact with a selectively absorbent stationary phase.
There are a number of different kinds of chromatography, which differ in the mobile and the stationary phase used.
Interesting Science Videos
What is Column Chromatography?
Column chromatography is a technique in which the substances to be separated are introduced onto the top of a column packed with an adsorbent, passed through the column at different rates that depend on the affinity of each substance for the adsorbent and for the solvent or solvent mixture, and are usually collected in solution as they pass from the column at different times.
It is a solid-liquid technique in which the stationary phase is a solid & the mobile phase is a liquid or gas.
It was developed by the American chemist D.T Day in 1900 while M.S. Tswett, the Polish botanist, 1906 used adsorption columns in his investigations of plant pigments.
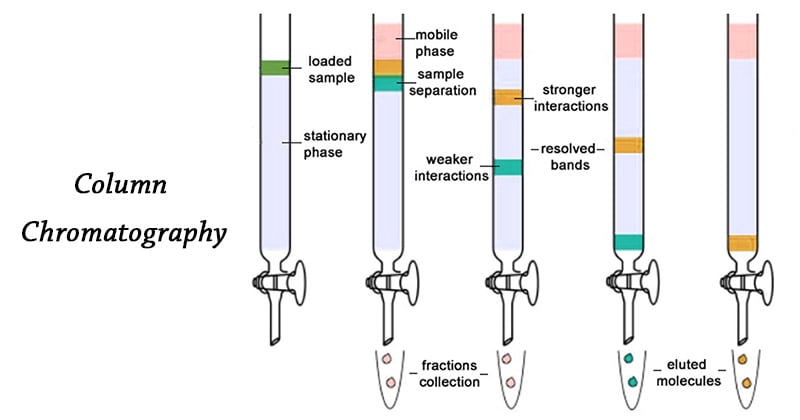
Image Source: PrepGenie.
Forms of Column Chromatography
There are two forms of column chromatography.
- Liquid chromatography (LC)
- Gas chromatography (GC)
The most widely used forms of column chromatography are:
- Adsorption chromatography
- Partition chromatography
- Ion exchange chromatography
- Gel chromatography
Principle of Column Chromatography
- In column chromatography the stationary phase is packed into a glass or metal column.
- The mixture of analytes is then applied and the mobile phase, commonly referred to as the eluent, is passed through the column either by use of a pumping system or applied gas pressure.
- The stationary phase is either coated onto discrete small particles (the matrix) and packed into the column or applied as a thin film to the inside wall of the column.
- As the eluent flows through the column the analytes separate on the basis of their distribution coefficients and emerge individually in the eluate as it leaves the column.
Instrumentation of Column Chromatography
A typical column chromatographic system using a gas or liquid mobile phase consists of the following components:
A stationary phase:
- Chosen to be appropriate for the analytes to be separated.
A column:
- In liquid chromatography these are generally 25- 50 cm long and 4mm internal diameter and made of stainless steel whereas in gas chromatography they are 1-3m long and 2- 4mm internal diameter and made of either glass or stainless steel.
- They may be either of the conventional type filled with the stationary phase, or of the microbore type in which the stationary phase is coated directly on the inside wall of the column.
A mobile phase and delivery system:
- Chosen to complement the stationary phase and hence to discriminate between the sample analytes and to deliver a constant rate of flow into the column.
An injector system:
- To deliver test samples to the top of the column in a reproducible manner.
A detector and chart recorder:
- To give a continuous record of the presence of the analytes in the eluate as it emerges from the column.
- Detection is usually based on the measurement of a physical parameter such as visible or ultraviolet absorption or fluorescence.
- A peak on the chart recorder represents each separated analyte.
A fraction collector: For collecting the separated analytes for further biochemical studies.
Steps in Column Chromatography
A. Preparation of the Column
- The column mostly consists of a glass tube packed with a suitable stationary phase.
- A glass wool/cotton wool or an asbestos pad is placed at the botton of the column before packing the stationary phase.
- After packing, a paper disc kept on the top, so that the stationary layer is not disturbed during the introduction of sample or mobile phase.
There are two types of preparing the column, they are:
1. Dry packing / dry filling
In this the required quantity of adsorbent is poured as fine dry powder in the column and the solvent is allowed to flow through the column till equilibrium is reached.
2. Wet packing / wet filling
In this, the slurry of adsorbent with the mobile phase is prepared and is poured into the column. It is considered as the ideal technique for packing.
- Before using column, it should be washed properly and dried.
- The column should also be free from impurity and uniformly filled with the stationary phase.
B. Introduction of the Sample
- The sample which is usually a mixture of components is dissolved in minimum quantity of the mobile phase.
- The entire sample is introduced into the column at once and get adsorbed on the top portion of the column.
- From this zone, individual sample can be separated by a process of elution.
C. Elution
- By elution technique, the individual components are separated out from the column.
- It can be achieved by two techniques:
- Isocratic elution technique: Same solvent composition or solvent of same polarity is used throughout the process of separation.
Eg. Use of chloroform alone.
- Gradient elution technique: Solvents of gradually ↑ polarity or ↑ elution strength are used during the process of separation.
E.g. initially benzene, then chloroform, then ethyl acetate then chloroform
D. Detection of Components
- If the compounds separated in a column chromatography procedure are colored, the progress of the separation can simply be monitored visually.
- If the compounds to be isolated from column chromatography are colorless.
- In this case, small fractions of the eluent are collected sequentially in labelled tubes and the composition of each fraction is analyzed by TLC.
Factors Affecting Column Efficiency
- Dimensions of the column
- Particle size of the adsorbent
- Nature of the solvent
- Temperature of the column
- Pressure
Applications
Column chromatography is one of the most useful methods for the separation and purification of both solids and liquids. Its major application includes:
- Separation of mixture of compounds.
- Removal of impurities or purification process.
- Isolation of active constituents.
- Isolation of metabolites from biological fluids.
- Estimation of drugs in formulation or crude extracts.
Advantages
- Any type of mixture can be separated by column chromatography.
- Any quantity of the mixture can also be separated.
- Wider choice of mobile phase.
- In preparative type, the sample can be separated and reused.
- Automation is possible.
Limitations
- Time consuming method.
- More amounts of solvents are required which may be expensive.
- Automation makes the technique more complicated and costly.
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://www.slideshare.net/shaisejacob/column-chromato
- https://chromatography.conferenceseries.com/events-list/applications-of-chromatography
- https://www.slideshare.net/RameshJupudi/column-chromatography-ppt
- http://library.umac.mo/ebooks/b28050630.pdf
- https://www.slideshare.net/krakeshguptha/column-chromatography-26966949
- https://en.wikipedia.org/wiki/Column_chromatography
- https://www.merriam-webster.com/medical/column%20chromatography

amazing content , really helpful in exam preparation
notes are very useful to undergraduates students who are in the field of chemitry and pharmacy
Really, it was very helpful. I understand it very well for first time…. Thanks
Yes, the website provide clear elocution about column chromatography!
Best notes
it is very helpful to me a very nice website