Chromatography is the separation of a mixture of compounds into their individual components based on their relative interactions with an inert matrix.
Interesting Science Videos
What is Ion Exchange Chromatography?
Ion exchange chromatography (or ion chromatography) is a process that allows the separation of ions and polar molecules based on their affinity to ion exchangers.
The principle of separation is thus by reversible exchange of ions between the target ions present in the sample solution to the ions present on ion exchangers.
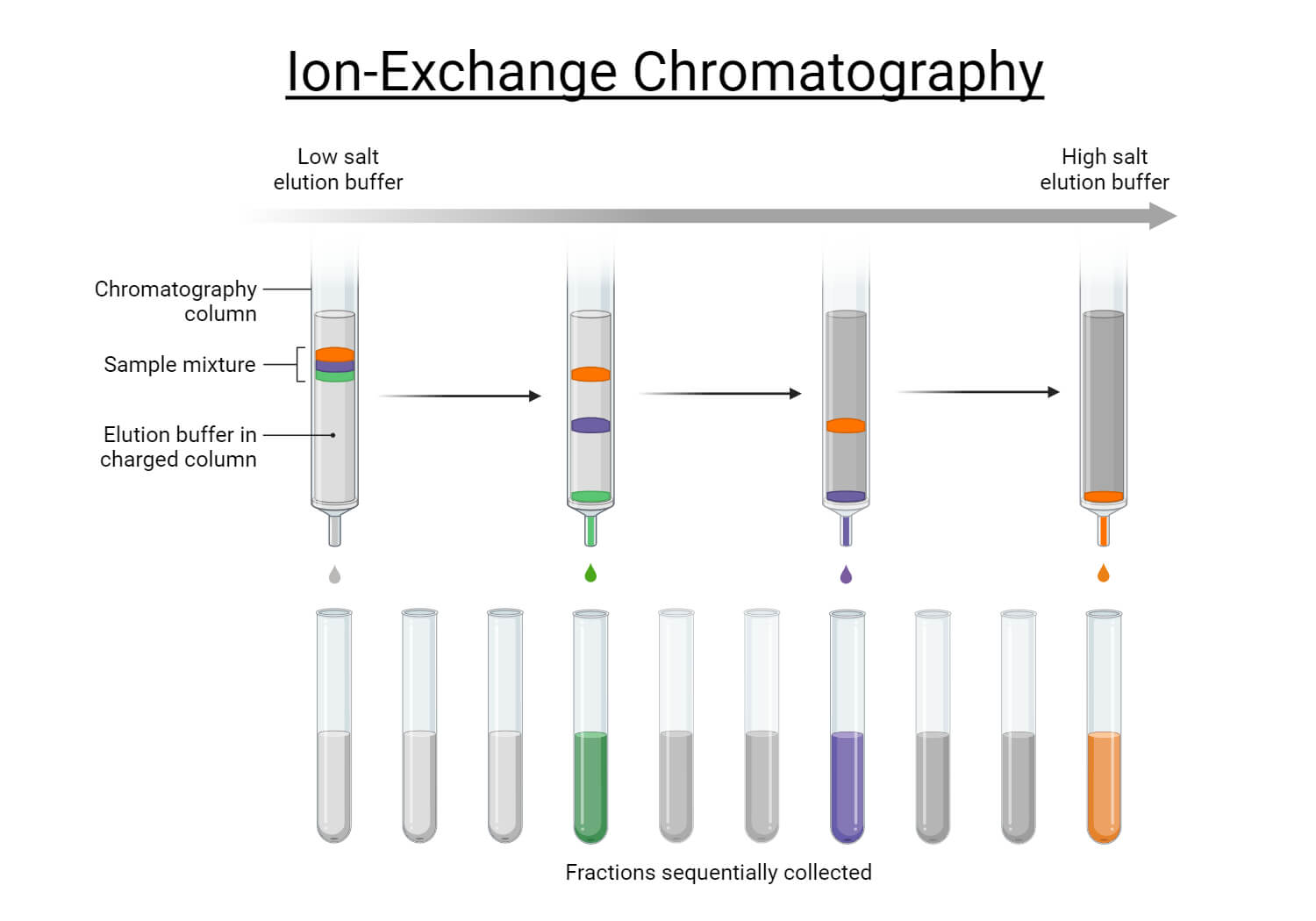
In this process, two types of exchangers i.e., cationic and anionic exchangers can be used.
- Cationic exchangers possess negatively charged group, and these will attract positively charged cations. These exchangers are also called “Acidic ion exchange” materials, because their negative charges result from the ionization of acidic group.
- Anionic exchangers have positively charged groups that will attract negatively charged anions. These are also called “Basic ion exchange” materials.
- Ion exchange chromatography is most often performed in the form of column chromatography. However, there are also thin-layer chromatographic methods that work basically based on the principle of ion exchange.
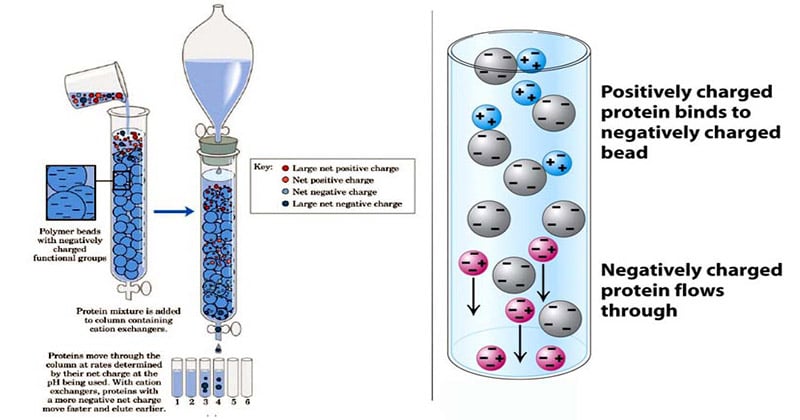
Image Source: Technology in Science
Working Principle of ion exchange chromatography
This form of chromatography relies on the attraction between oppositely charged stationary phase, known as an ion exchanger, and analyte.
- The ion exchangers basically contain charged groups covalently linked to the surface of an insoluble matrix.
- The charged groups of the matrix can be positively or negatively charged.
- When suspended in an aqueous solution, the charged groups of the matrix will be surrounded by ions of the opposite charge.
- In this “ion cloud”, ions can be reversibly exchanged without changing the nature and the properties of the matrix.
Instrumentation of ion exchange chromatography
Typical IC instrumentation includes: pump, injector, column, suppressor, detector and recorder or data system.
- Pump
The IC pump is considered to be one of the most important components in the system which has to provide a continuous constant flow of the eluent through the IC injector, column, and detector.
- Injector
Sample introduction can be accomplished in various ways. The simplest method is to use an injection valve. Liquid samples may be injected directly and solid samples need only to be dissolved in an appropriate solvent. Injectors should provide the possibility of injecting the liquid sample within the range of 0.1 to 100 ml of volume with high reproducibility and under high pressure (up to the 4000 psi).
- Columns
Depending on its ultimate use and area of application, the column material may be stainless steel, titanium, glass or an inert plastic such as PEEK. The column can vary in diameter from about 2mm to 5 cm and in length from 3 cm to 50 cm depending on whether it is to be used for normal analytical purposes, microanalysis, high speed analyses or preparative work.
Guard column is placed anterior to the separating column. This serves as a protective factor that prolongs the life and usefulness of the separation column. They are dependable columns designed to filter or remove particles that clog the separation column
- Suppressor
The suppressor reduces the background conductivity of the chemicals used to elute samples from the ion-exchange column which improves the conductivity measurement of the ions being tested. IC suppressors are membrane-based devices which are designed to convert the ionic eluent to water as a means of enhancing the sensitivity.
- Detectors
Electrical conductivity detector is commonly use.
- Data system
In routine analysis, where no automation is needed, a pre-programmed computing integrator may be sufficient. For higher control levels, a more intelligent device is necessary, such as a data station or minicomputer.
Procedure of ion exchange chromatography
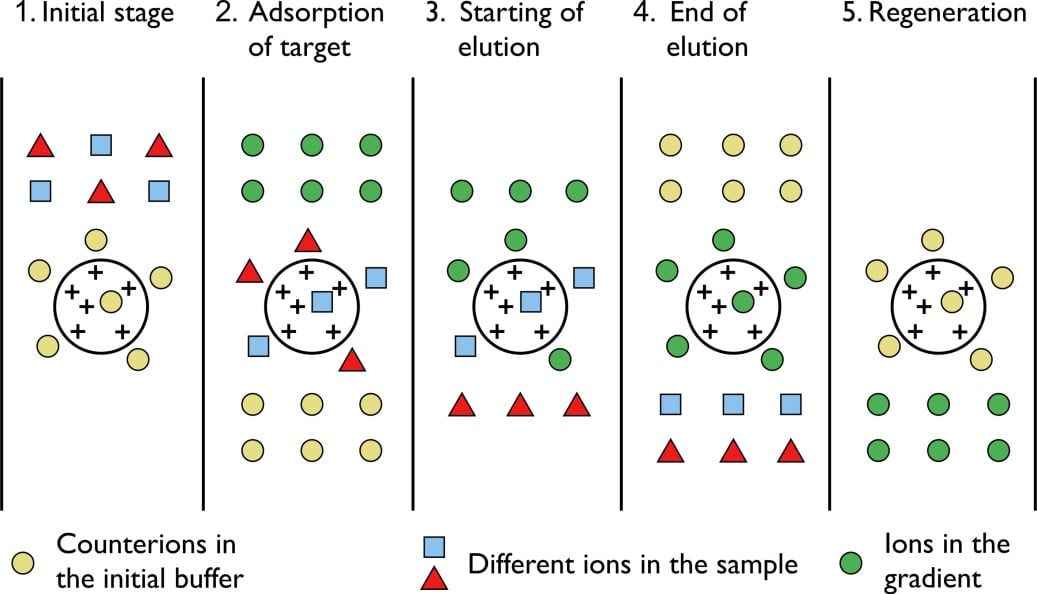
- Ion exchange separations are carried out mainly in columns packed with an ion-exchanger.
- These ionic exchangers are commercially available. They are made up of styrene and divinyl benzene. Example. DEAE-cellulose is an anionic exchanger, CM-cellulose is a cationic exchanger.
- The choice of the exchanger depends upon the charge of particle to be separated. To separate anions “Anionic exchanger” is used, to separate cations “Cationic exchanger” is used.
- First the column is filled with ion exchanger then the sample is applied followed by the buffer. The tris-buffer, pyridine buffer, acetate buffer, citrate and phosphate buffers are widely used.
- The particles which have high affinity for ion exchanger will come down the column along with buffers.
- In next step using corresponding buffer separates the tightly bound particles.
- Then these particles are analyzed spectroscopically.
Applications of ion exchange chromatography
- An important use of ion-exchange chromatography is in the routine analysis of amino acid mixtures.
- The 20 principal amino acids from blood serum or from the hydrolysis of proteins are separated and used in clinical diagnosis.
- This is most effective method for water purification. Complete deionization of water (or) a non-electrolyte solution is performed by exchanging solute cations for hydrogen ions and solute anions for hydroxyl ions. This is usually achieved by method is used for softening of drinking water.
- In the analysis of products of hydrolysis of nucleic acids. In this way, information is gained about the structure of these molecules and how it relates to their biological function as carriers of hereditary information.
- Chelating resins are used to collect trace metals from seawater.
- To analyze lunar rocks and rare trace elements on Earth.
Advantages of ion exchange chromatography
- It is one of the most efficient methods for the separation of charged particles.
- It can be used for almost any kind of charged molecule including large proteins, small nucleotides and amino acids.
- Ion exchange is used for both analytical and preparative purposes in the laboratory, the analytical uses being the more common.
- Inorganic ions also can be separated by ion-exchange chromatograph.y
Limitations of ion exchange chromatography
- Only charged molecules can be separated.
- Buffer Requirement
References
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology (8 eds.). Cambridge University Press: New York.
- https://www.biochemden.com/ion-exchange-chromatography/
- https://www.britannica.com/science/ion-exchange-reaction/Applications-of-ion-exchange
- http://elte.prompt.hu/sites/default/files/tananyagok/IntroductionToPracticalBiochemistry/ch06s02.html
- http://cdn.intechopen.com/pdfs/43603/InTech-Ion_exchange_chromatography_an_overview.pdf

Which soln is used for mobile phase?
Which column are used and how column work
It was excellent👍
This document is more intellectual and deserve to be valuable at all,
Thank you for your support.
Gud for all
It was excellent material to study and also easy to understanding as well as clearly explained everything. Thank you so much.
It’s super cool notes to study and also easy than class note. The expansions were can easily catch to everyone simply and understand simply. Thanku so much.
It was amazing to study this..Very simple language as well as clearly explained everything….Shared with my friends also…And they loved the content…Thank you so much….
Yes