When it comes to the biochemical activities and enzyme systems, each bacterial genus has its unique characteristics. These unique characteristics are the basis for differentiating and identifying bacteria based on their biochemical properties.
Carbohydrates like glucose, lactose, and sucrose are simple sugar but different bacteria utilize them producing different end products and some may not even utilize one or all of these sugars. Some ferment these sugars and release acid and gas as byproducts while some do not.
The triple sugar iron agar (TSIA) test is a biochemical test used to differentiate bacteria based on their ability to ferment these three sugars and release acid and hydrogen sulfide gas.
In 194 S. Edward Sulkin, and Joshep C. Willett first described the TSIA medium as a selective and differential medium to differentiate bacteria, especially of the Enterobacteriaceae family. Hajna A.A. modified it in 1945 and since then is still used in microbiology laboratories as a major tool, primarily for differentiation and presumptive identification of Gram-negative enteric pathogens.
TSIA test is similar to Kligler’s Iron Agar (KIA) test except that the TSIA medium contains ‘1% sucrose’ as extra sugar in addition to glucose and lactose in the KIA medium.
Interesting Science Videos
Objectives of TSIA Test
- To differentiate Gram-negative enteric bacilli.
- To test the bacteria’s ability to utilize glucose, lactose, and/or sucrose and produce H2S gas.
- To differentiate lactose fermenters from non-lactose fermenters.
Principle of TSIA Test
TSIA test is based on the distinct metabolic pattern of the different bacterial genera to metabolize glucose, lactose, sucrose, and sodium thiosulfate (a sulfur compound). Fermentation of these sugars will produce acid decreasing the pH of the medium and turning it to red color. Similarly, the metabolism of sodium thiosulfate is indicated by turning the medium black.
Glucose will initially be fermented by bacteria capable of doing so, producing metabolic acids. This resulting acid will lower the pH of the medium, turning the slant and butt regions yellow. But because there isn’t much glucose left, it will quickly run out.
If the bacteria are lactose and or sucrose non-fermenter, they won’t be able to use lactose and sucrose as a carbon source. Therefore, once the glucose is exhausted, the oxidative metabolism of peptone in the slant will be triggered. This oxidative metabolism will increase the pH of the medium to an alkaline level and turns the yellow slant to red. However, because there won’t be any to very little oxygen in the butt, peptone won’t undergo oxidative degradation there, leaving the butt’s color unchanged. Thus the bacteria that do not ferment lactose and/or sucrose but ferment glucose will develop a red slant and yellow butt (written as Red/Yellow or K/A).
In contrast, if the bacteria are lactose and/or sucrose fermenters, they will begin to ferment these sugars and release acid. The released acid will drop the pH of the medium and the indicator will turn the medium yellow in both the slant and butt area. As there is abundant carbohydrate (sucrose and lactose), the oxidative metabolism of peptone won’t be triggered. Thus bacteria that ferment the lactose and/or sucrose (or all three sugars) will develop a yellow slant and yellow butt (written as Yellow/Yellow or A/A).
If the bacteria are not able to ferment any of the sugars, the medium will remain red (written as Red/Red or K/K).
Bacteria that produce H2S can break down the sodium thiosulfate found in culture media and cause it to decrease, generating H2S gas. The resulting H2S gas subsequently interacts with the ferric ions to produce water-insoluble ferrous sulfide, which is black in color. The presence of H2S is indicated by this insoluble black-colored molecule turning the culture media black.
The creation of gas bubbles above the medium or breaking or displacement of the medium are signs that gas is being produced during the fermentation of sugar.
Requirements for TSIA Test
1. Culture Media
Triple sugar iron agar medium is used for the TSIA test.
Composition of TSIA Medium per 1000 mL
Peptone – 20.00 grams
HM Extract (Meat Extract) – 3.00 grams
Yeast Extract – 3.00 grams
Dextrose (Glucose) – 1.00 grams
Lactose – 10.00 grams
Sucrose – 10.00 grams
Sodium Chloride – 5.00 grams
Ferric Citrate – 0.300 grams
Sodium Thiosulfate – 0.300 grams
Phenol Red – 0.024 grams
Agar – 12.00 grams
Final pH – 7.4±0.2 at 25°C
Preparation of TSIA Medium Slant
- Measure the appropriate amount of TSIA media powder (64.62 grams per 1000 mL) and dissolve it in the water of the required volume in a conical flask (or glass bottle).
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve entirely in water.
- Dispense about 5 to 7 mL of the medium in each test tube and loosely put on the cap or cotton plug the opening.
- Autoclave the tubes at 121°C and 15 lbs pressure for 15 minutes.
- Let it cool and solidify at a slanting position (around 30° inclinations) to form an agar slant with a deep butt of about 2.5 to 5 cm.
2. Reagents
No additional reagents are required.
3. Equipment
| Test tubes Incubator | Weighing Machine Autoclave | Bunsen Burner | Inoculating Loop |
PPE and other general laboratory materials
4. Sample Organism (Test Bacteria)
5. Control Organism
Escherichia coli ATCC 25922
Citrobacter freundii ATCC 8090
Proteus vulgaris ATCC 6380:
Salmonella Paratyphi A ATCC 9150
Salmonella Typhimurium ATCC 14028
Shigella flexneri ATCC 12022
Pseudomonas aeruginosa ATCC 27853
Procedure of TSIA Test
- Touch a well-isolated colony from a fresh culture of the test bacterium that is 18 to 24 hours old using a sterile inoculating wire.
- Stab the butt up to 3 to 5 mm above the base of the test tube using the inoculating wire and while withdrawing, streak the slant.
- Incubate the tube aerobically (with a loose cap) at 35±22°C for about 24 hours.
- Examine for color change of the slant and butt and report the color within 24 hours of incubation. (If you want to read the H2S production, incubate it for another 24 to 48 hours, but read sugar fermentation and color change within the first 24 hours of inoculation and incubation.)
Result and Interpretation of TSIA Test
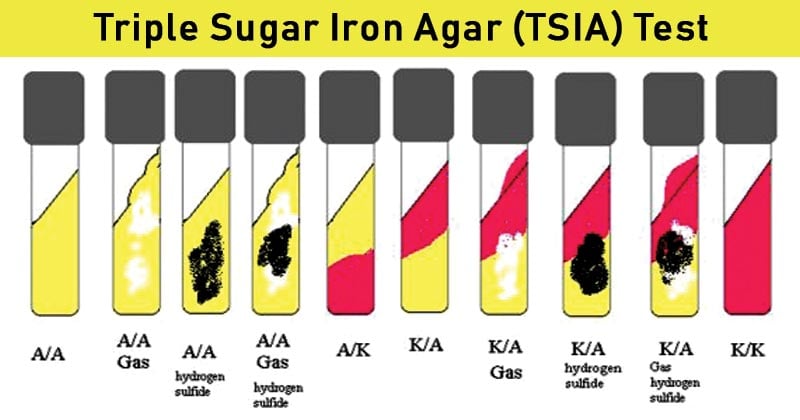
- The red slant and yellow butt (Red/Yellow or Alkaline (K)/Acidic (A)) indicate only glucose is fermented.
- Yellow slant and yellow butt (Yellow/Yellow or Acidic (A)/Acidic (A)) indicate lactose and/or sucrose fermentation or fermentation of all three sugars.
- Red slant and red butt (Red/Red or Alkaline (K)/Alkaline (K)) indicate none of the three sugars are fermented.
- Blackening of the media or formation of the black-colored spots indicates H2S production.
- Cracking of the media, gas bubbles of the media, or forming a gap in the media indicates gas production.
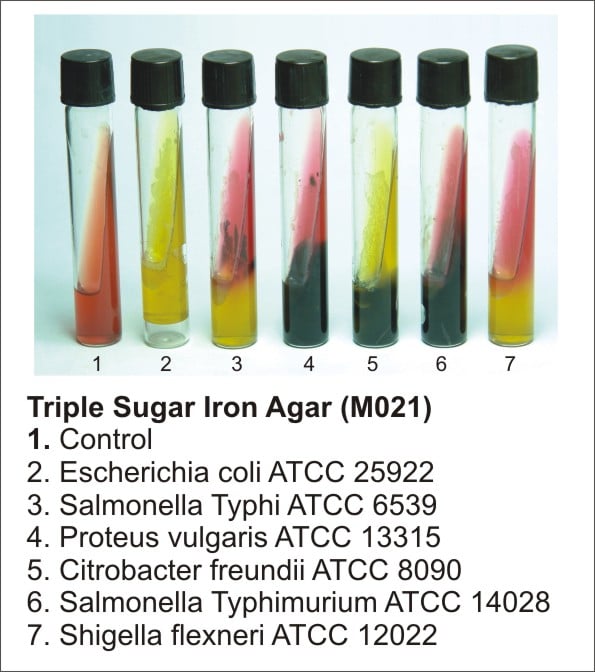
Quality Control
The above control organisms will show the following results:
Escherichia coli ATCC 25922: Yellow/Yellow, Gas +ve, H2S – ve
Citrobacter freundii ATCC 8090: Yellow/Yellow, Gas +ve, H2S + ve
Proteus vulgaris ATCC 6380: Red/Yellow, Gas – ve, H2S + ve
Salmonella Paratyphi A ATCC 9150: Red/Yellow, Gas +ve, H2S – ve
Salmonella Typhimurium ATCC 14028: Red/Yellow, Gas +ve, H2S + ve
Shigella flexneri ATCC 12022: Red/Yellow, Gas – ve, H2S – ve
Pseudomonas aeruginosa ATCC 27853: Red/Red, Gas – ve, H2S – ve
TSIA Test Results of Some Common Enteric Pathogens
| Name of Bacteria | Color of Slant/Butt (pH of Slant/Butt) | H2S Production | Gas Production |
| E. coli | Yellow/Yellow (Acidic/Acidic) | – ve | + ve |
| K. pneumoniae | Yellow/Yellow (Acidic/Acidic) | – ve | + ve |
| K. oxytoca | Yellow/Yellow (Acidic/Acidic) | – ve | + ve |
| Shigella spp. | Red/Yellow (Alkaline/Acidic) | – ve | – ve |
| Serratia marcescens | Red/Yellow (Alkaline/Acidic) | – ve | Variable |
| Salmonella Typhi | Red/Yellow (Alkaline/Acidic) | – ve | + ve |
| Yersinia enterocolitica | Red/Yellow (Alkaline/Acidic) | – ve | Variable |
| Enterobacter cloacae | Yellow/Yellow (Acidic/Acidic) | – ve | + ve |
| Salmonella Paratyphi B and C | Red/Yellow (Alkaline/Acidic) | + ve | + ve |
| Providencia stuartii | Red/Yellow (Alkaline/Acidic) | – ve | – ve |
| Vibrio cholerae | Red/Yellow or, Yellow/Yellow (variable lactose fermentation) | – ve | – ve |
Precautions
- If the medium is broken or has a gap in it before inoculation, do not use the medium as it may give false results about gas production.
- Avoid stabbing with an inoculating loop because it may produce cracking and confusion when reporting the production of gas.
- Before 18 hours of incubation, do not read the sugar utilization result since the glucose may not have been completely utilized and thus the oxidative metabolism of the peptones may not have commenced.
- Do not read the result long after 24 hours because the whole tube may turn black and fails to show the color change of the medium.
- Remove the tubes from the incubator and store them in a freezer at 4°C if you need to read the result after 24 hours.
Applications of TSIA Test
- Presumptive identification of enteric pathogens, particularly Gram-negative bacilli
- Differentiation of members of Enterobacteriaceae
- Detection of lactose fermenter, non-lactose fermenter, and H2S producer.
- An important tool in biochemically identifying bacteria in research or diagnostic laboratory
Limitations of TSIA Test
- It is only a presumptive differential test; hence requires other tests for conformation of bacterial identification.
- It is advised to read the results within 18 to 24 hours – neither too soon nor too late. This rigorous time frame limits the time of reading results because otherwise, we can get false results.
- It is not suitable for all fecal bacteria. For example, in the case of mixed flora, the result won’t be accurate, both E. coli and Klebsiella spp. will produce similar results, etc.
- H2S production may be inhibited for sucrose fermenters.
- We can’t differentiate whether lactose or sucrose alone are fermented or both of them together.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition) . Washington, DC : ASM Press 1752 N St., N.W., [2016] doi:10.1128/9781555818814.ch3.17.25
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.) P. 183 – 185. St. Louis, Missouri: Elsevier
- S. Edward Sulkin and Joseph C. Willett. (1940). A triple sugar-ferrous sulfate medium for use in identification of enteric organisms. Journal of Laboratory and Clinical Medicine. Vol. 25, P-649 – 653.
- Hajna, A. A. (1945). Triple-Sugar Iron Agar Medium for the Identification of the Intestinal Group of Bacteria. Journal of Bacteriology, 49(5), 516-517. https://doi.org/10.1128/jb.49.5.516-517.1945
- https://microbiologie-clinique.com/triple-sugar-iron-agar.html
- https://microbiologyinfo.com/triple-sugar-iron-tsi-test/
- https://www.austincc.edu/microbugz/triple_sugar_iron_agar.php
- https://www.onlinebiologynotes.com/tsi-triple-sugar-iron-test-objective-principle-procedure-and-result/
- TSI slant. (2021, December 9). In Wikipedia. https://en.wikipedia.org/wiki/TSI_slant
- https://microbeonline.com/triple-sugar-iron-agar-tsi-principle-procedure-and-interpretation/
- https://asm.org/ASM/media/Protocol-Images/Triple-Sugar-Iron-Agar-Protocols.pdf?ext=.pdf
- https://www.thomassci.com/Laboratory-Supplies/Microbiological-Media/_/Triple-Sugar-Iron-Agar2
- https://www.himedialabs.com/media/TD/M021I.pdf
- https://asm.org/ASM/media/Protocol-Images/Triple-Sugar-Iron-Agar-Protocols.pdf?ext=.pdf

What is the meaning of “+Ve” , “-Ve” anda variabel ?
This piece is quite concise and understandable.
This is great and educative
Yes, you’re right dear!