Different bacteria have different enzyme systems and different biochemical activities to derive metabolic energy. Based on the enzyme system and biochemical characteristics, the end product of metabolism of the same substrate may vary between different bacterial species. Some bacteria can ferment carbohydrates like glucose and lactose and release acid, while some can only ferment one or even none of these sugars. During the fermentation of these sugars, some species release gas while some do not.
Kligler’s Iron Agar (KIA) Test is the biochemical test to assess the different abilities of bacteria to either ferment glucose and/or lactose in a different way or lack of ability to ferment them at all together with the ability to produce H2S gas.
Kligler in 1918, first formulate a medium, called Kligler’s lead acetate medium, to detect H2S production and differentiation of Salmonella and Shigella spp. It was combined with Russel’s Double Sugar Agar Medium to differentiate enteric bacilli. Later, the combined medium was modified by Bailey and Lacey by replacing the Andrade indicator with phenol red and formulating a new medium called Kligler’s Iron Agar medium, and devised the KIA test.
The KIA test uses KIA (Kligler’s Iron Agar) medium to differentiate the bacteria based on their ability to produce H2S, produce gas, and produce acid by fermenting either glucose or lactose or both. This test is mainly used to differentiate Gram-negative enteric bacilli in clinical or non-clinical samples.
This test is similar to the TSI test (Triple Sugar Iron Test) except that the TSI agar in the TSI test contains a third sugar, sucrose, so it will not differentiate lactose fermenter and non-lactose fermenter because the result will be the same if the bacteria ferment the sucrose or the lactose or both.
Interesting Science Videos
Objectives of KIA Test
- To differentiate Gram-negative enteric bacilli
- To test the bacteria’s ability to produce H2S, produce gas, and produce acid by fermenting either glucose or lactose or both
- To differentiate lactose fermenters from non-lactose fermenters
Principle of KIA Test
- Different bacteria have different abilities to use glucose, lactose, and sulfur compounds.
- Bacteria capable of fermenting glucose will initially ferment the glucose, releasing metabolic acids. Thus produced acid will decrease the pH of the medium making it yellow-colored in both the slant and butt area. However, as the amount of glucose is very low, it will soon be depleted.
- If the bacteria are lactose non-fermenter, they won’t be able to use lactose as a carbon source, and the depletion of glucose will trigger oxidative metabolism of peptone in the slant. This oxidative metabolism will increase the pH of the medium to an alkaline level and turns the yellow color of the yellow slant to red. But, as there will be no to very less oxygen in the butt, oxidative breakdown of peptone won’t occur there and the color of the butt will remain yellow. Thus the bacteria that do not ferment lactose but ferment glucose will develop a red slant and yellow butt (written as Red/Yellow or K/A).
- If the bacteria are lactose fermenters, the bacteria will begin to ferment the lactose and release acid. The released acid will drop the pH of the medium and the indicator will turn the medium yellow in both the slant and butt area. As there is abundant lactose, the oxidative metabolism of peptone won’t be triggered. Thus bacteria that ferment the lactose (either lactose only or both the lactose and glucose) will develop a yellow slant and yellow butt (written as Yellow/Yellow or A/A).
- If the bacteria are not able to ferment both of the sugars, the medium will remain red (written as Red/Red or K/K).
- The gas production during sugar fermentation will be indicated by the formation of gas bubbles over the medium or cracking/displacement of the medium.
- H2S-producing bacteria can metabolize the sodium thiosulfate present in the culture media and reduce it releasing the H2S gas. The produced H2S gas then reacts with the ferric ions forming water-insoluble black-colored ferrous sulfide. This insoluble black-colored compound turns the culture media black indicating the H2S production.
Requirements for KIA Test
a. Culture Media
Kligler’s Iron Agar (KIA) medium is used during the test.
Composition of the KIA Medium per 1000 mL
Peptone- 15.00 grams
HM Peptone B (Beef Extract)- 3.00 grams
Proteose Peptone- 5.00 grams
Yeast Extract- 3.00 grams
Dextrose (Glucose)- 1.00 grams
Lactose- 10.00 grams
Sodium Chloride- 5.00 grams
Ferrous Sulfate- 0.200 grams
Sodium Thiosulfate- 0.300 grams
Phenol Red- 0.024 grams
Agar- 15.00 grams
Final pH 7.4±0.2 at 25°C
(Reference: Kligler Iron Agar (himedialabs.com))
Preparation of the KIA Medium
- Measure the appropriate amount of KIA media powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company (57.52 grams per 1000 mL).
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- Dispense about 5 to 7 mL of the medium in each test tube and loosely put on the cap or cotton plug the opening.
- Autoclave the tubes at 121°C and 15 lbs pressure for 15 minutes.
- Let it cool and solidify at a slanting position (around 30° inclinations) to form an agar slant with a deep butt.
b. Reagents
No reagents are required for the KIA test.
c. Equipment
| Test tubes Incubator | Weighing Machine Autoclave | Bunsen burner | Inoculating loop |
PPE and other general laboratory materials
d. Test Organism (Sample Bacteria)
e. Control Organisms
Escherichia coli ATCC 25922
Citrobacter freundii ATCC 8090
Proteus vulgaris ATCC 6380:
Salmonella Paratyphi A ATCC 9150
Salmonella Enteritidis ATCC 13076
Shigella flexneri ATCC 12022
Pseudomonas aeruginosa ATCC 27853
Procedure of KIA Test
- Using a sterile inoculating wire, touch a well-isolated colony from fresh culture (18 to 24 hours old) of the test bacterium.
- Inoculate the KIA medium tube by stabbing the butt up to 3 to 5 mm above the base of the tube and while withdrawing, streak the slant portion.
- Incubate the tube aerobically (with a loose cap) at 35±22°C for about 24 hours.
- Examine for color change of the slant and butt and report the color within 24 hours of incubation. (If you want to read the H2S production, incubate it for other 24 to 48 hours, but read sugar fermentation and color change within the first 24 hours of inoculation and incubation.)
Result and Interpretation of KIA Test
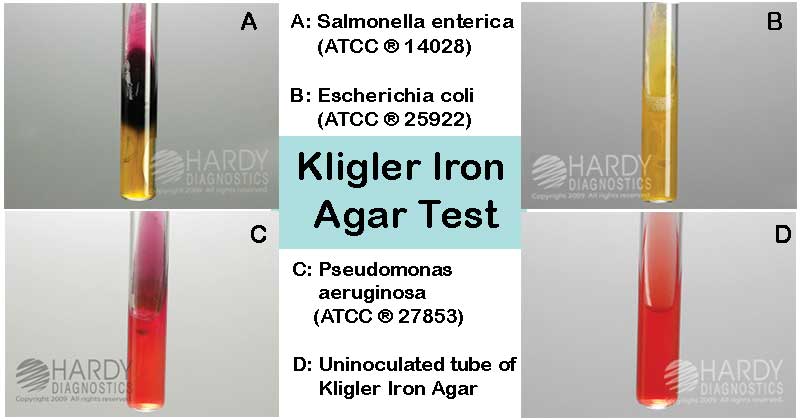
- Red slant and yellow butt (Red/Yellow or Alkaline (K)/Acidic (A)) indicate glucose fermenter and lactose non-fermenter.
- Yellow slant and yellow butt (Yellow/Yellow or Acidic (A)/Acidic (A)) indicate lactose fermentation (either lactose alone or both lactose and glucose fermentation).
- Red slant and red butt (Red/Red or Alkaline (K)/Alkaline (K)) indicate both lactose and glucose non-fermenter.
- Blackening of the media or formation of the black-colored spots indicates H2S production.
- Cracking of the media, gas bubbles of the media, or formation of a gap in the media indicates gas production.
Quality Control
Incubate the TSI agar medium with the control organisms describes above and read the result as:
Escherichia coli ATCC 25922: Yellow/Yellow, Gas +ve, H2S – ve
Citrobacter freundii ATCC 8090: Yellow/Yellow, Gas +ve, H2S + ve
Proteus vulgaris ATCC 6380: Red/Yellow, Gas – ve, H2S + ve
Salmonella Paratyphi A ATCC 9150: Red/Yellow, Gas +ve, H2S – ve
Salmonella Enteritidis ATCC 13076: Red/Yellow, Gas +ve, H2S + ve
Shigella flexneri ATCC 12022: Red/Yellow, Gas – ve, H2S – ve
Pseudomonas aeruginosa ATCC 27853: Red/Red, Gas – ve, H2S – ve
KIA Test Results of Some Common Enteric Pathogens
| Name of Bacteria | Color of Slant/Butt(pH of Slant/Butt) | H2S Production | Gas Production |
| E. coli | Yellow/Yellow(Acidic/Acidic) | – ve | + ve |
| K. pneumoniae | Yellow/Yellow(Acidic/Acidic) | – ve | + ve |
| K. oxytoca | Yellow/Yellow(Acidic/Acidic) | – ve | + ve |
| Shigella spp. | Red/Yellow(Alkaline/Acidic) | – ve | – ve |
| Serratia marcescens | Red/Yellow(Alkaline/Acidic) | – ve | Variable |
| Salmonella Typhi | Red/Yellow(Alkaline/Acidic) | – ve | + ve |
| Yersinia enterocolitica | Red/Yellow(Alkaline/Acidic) | – ve | Variable |
| Enterobacter cloacae | Yellow/Yellow(Acidic/Acidic) | – ve | + ve |
| Salmonella Paratyphi B and C | Red/Yellow(Alkaline/Acidic) | + ve | + ve |
| Providencia stuartii | Red/Yellow(Alkaline/Acidic) | – ve | – ve |
| Vibrio cholerae | Red/YellowOr, Yellow/Yellow (variable lactose fermentation) | – ve | – ve |
Precautions
- Do not use if the medium is cracked or if there is a gap in the medium prior to inoculation.
- Don’t use an inoculating loop for stabbing because it can create cracking which will confuse during reporting the gas production.
- Do not read the result for sugar utilization prior to 18 hours of incubation because the glucose may not be depleted and the oxidative metabolism of peptones may not be started yet.
- Do not read the result long after 24 hours because the whole tube may turn black and fails to show the color change of the medium.
- If you have to read the result after 24 hours, remove the tubes from the incubator and store them in a freeze at 4°C.
Applications of KIA Test
- Identification of Gram-negative bacilli
- To identify and differentiate members of Enterobacteriaceae
- Differentiation of Salmonella spp. and Shigella spp.
- Differentiating lactose fermenters and non-lactose fermenters
- Detection of H2S production
- Screening fecal cultures
Limitations of KIA Test
- It has a strict time frame, and it is recommended to read the result within 18 to 24 hours, not too early and not too late.
- Lactose fermentation alone and together with glucose gives the same result. So, is not ideal for differentiating based on lactose and glucose fermentation ability.
- H2S will turn the medium black, so there may be a problem in noticing the color of the slant and butt.
- It is not a confirmatory test; hence, it needs other biochemical tests’ results for complete identification.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier
- Kligler’s Iron Agar (KIA): Principle, Procedure, Results • Microbe Online
- Kligler’s Iron Agar Test – Procedure, Uses and Interpretation (microbiologyinfo.com)
- Kligler’s Iron Agar Test Principle, Procedure, Result (microbiologynote.com)
- Kligler iron agar (KIA): composition, preparation, uses and interpretation of result – Online Biology Notes
- Kligler Iron Agar Test: Principle, Procedure, and Result (researchtweet.com)
- Kligler Iron Agar Test Flashcards | Quizlet

Concise and comprehensive!Thanks