Hydrogen Sulfide (H2S) Test is the biochemical test used to detect the ability of bacteria to produce H2S gas.
Several bacteria and archaea can metabolically reduce sulfur-containing compounds to hydrogen sulfide and derive energy during the process. These sulfur-containing compounds may be inorganic compounds like sulfate, sulfite, thiosulfate, or tetrathionates, or maybe organic sulfur-containing compounds like sulfur amino acids and proteins, or even elemental sulfur itself. Sulfur from these compounds is enzymatically reduced and liberated as hydrogen sulfide (H2S) gas. A wide range of aerobic, facultative, and anaerobic bacterial species have the capacity to produce H2S gas, and they are collectively called hydrogen sulfide-producing bacteria.
Several chemolithotrophs, autotrophs, and heterotrophs in the environment can use sulfur compounds to produce metabolic energy and decompose organic compounds or recycle inorganic compounds. Besides, several commensals and pathogenic bacterial species are also able to produce H2S. Hence, detecting the ability of bacteria to produce H2S gas is an important biochemical test used in microbiology labs and diagnostic labs for the identification of unknown bacteria.
It is an important method to detect coliforms in water, identify fecal pathogens, differentiate enteric pathogenic bacteria, and characterize other pathogenic or non-pathogenic bacterial isolates.
H2S gas is a colorless, toxic, corrosive, and flammable reduced gas form of sulfur having a peculiar rotten egg-like smell. Microbial production of H2S is detected by allowing it to react with ferric ions or lead acetate to produce black-colored ferrous sulfide or lead sulfide respectively. In the H2S test, sodium thiosulfate is mainly used as a sulfur source. Alternatively, some media also contains peptones, cysteine, and sulfites as sulfur sources. As an indicator to detect the production of H2S gas, ferrous sulfate, ferric ammonium citrate, ferric citrate, peptonized iron, and lead acetate is used in H2S detecting medium. Among these, lead acetate is the most sensitive and has the ability to detect even the trace amount of H2S, but, since most of the common pathogenic bacteria produce abundant H2S gas, cheap, less toxic, and easy-to-use but less sensitive ferrous sulfate is widely incorporated in H2S detecting medium.
Interesting Science Videos
Objectives of Hydrogen Sulfide (H2S) Test
- To detect the ability of bacteria to produce H2S gas.
- To presumptively identify bacterial isolates.
Principle of Hydrogen Sulfide (H2S) Test
H2S-producing bacteria can metabolize the sulfur-containing compounds (mostly sodium thiosulfate) present in the culture media and reduce it releasing the H2S gas. The produced H2S gas then reacts with the ferric ions or lead acetate forming water-insoluble black-colored ferrous sulfide or lead sulfide respectively. This insoluble black-colored compound turns the culture media black indicating a positive result for the H2S production test (or H2S production).
Requirements for Hydrogen Sulfide (H2S) Test
a. Culture Media Used for H2S Test
A wide variety of culture media are available for the detection of hydrogen sulfide production. For tube H2S test, SIM (Sulfide Indole Motility) medium, KIA (Kligler’s Iron Agar), TSI (Triple Sugar Iron) Agar Medium, and Lead Acetate (LA) Agar are commonly used.
For the petri plate method, SS (Salmonella-Shigella) Agar, DCA (Deoxycholate citrate agar), BS (Bismuth Sulfite) medium, XLD (Xylose-lysine deoxycholate) agar medium, and HE (Hektoen Enteric) Agar are commonly used.
Nutrient broth, peptone water, tryptic soy broth, or other suitable broth media are used to inoculate bacteria for performing the lead acetate paper method.
| S/N | Preferred Culture Media | Sulfur Source | Indicator to Detect H2S |
| 1. | SIM Medium | Sodium thiosulfate | Peptonized iron |
| 2. | KIA Medium | Sodium thiosulfate | Ferrous sulfate |
| 3. | TSI Medium | Sodium thiosulfate | Ferrous sulfate |
| 4. | SS Medium | Sodium thiosulfate | Ferric citrate |
| 5. | DCA Medium | Proteose peptones | Ferric ammonium citrate |
| 6. | HE Medium | Sodium thiosulfate | Ferric ammonium citrate |
| 7. | BS Medium | Sulfite | Ferrous sulfate |
| 8. | XLD Medium | Sodium thiosulfate | Ferric ammonium citrate |
In this article, we will use SIM agar medium. It is a general-purpose medium and is suitable for the detection of H2S production, indole production, and motility of organisms.
Composition of SIM Medium per 1000 mL
HM Peptone B (Beef Extract)- 3.00 grams
Peptone- 30.0 grams
Peptonized Iron- 0.020 grams
Sodium thiosulfate- 0.025 grams
Agar- 3.00 grams
Final pH 7.3 ±0.2 at 25°C
(Reference: SIM Medium (himedialabs.com))
Preparation of SIM Medium
- Measure the appropriate amount of SIM agar powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company (36.23 grams per 1000 mL in the above media).
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- Dispense about 5 mL of the medium in each test tube and loosely put on the cap or cotton plug the opening.
- Autoclave the tubes at 121°C and 15 lbs pressure for 15 minutes.
- Let it cool and solidify at an upright position (forming butt only).
For the plate method, we will be using Hektoen Enteric (HE) agar medium. The plate method is generally used for the isolation of fecal pathogens and enteric bacteria from food/beverage/water samples. HE is suitable for every enteric pathogenic and non-pathogenic organism (almost all Enterobacteriaceae members). In the clinical lab, DCA and SS agar are preferred over HE agar if the objective is to isolate and differentiate Salmonella spp. and Shigella spp., but they inhibit several other Enterobacteriaceae; hence we are using HE agar medium.
Composition of HE Agar per 1000 mL
Proteose peptone- 12.0 grams
Yeast Extract- 3.00 grams
Lactose- 12.0 grams
Sucrose- 12.0 grams
Salicin- 2.00 grams
Bile Salt Mixtures- 9.00 grams
Sodium Chloride- 5.00 grams
Sodium Thiosulfate- 5.00 grams
Ferric Ammonium Citrate- 1.50 grams
Acid Fuchsin- 0.10 grams
Bromothymol Blue- 0.065 grams
Agar- 15.0 grams
Final pH 7.5 ±0.2 at 25°C
(Reference: Hektoen Enteric Agar (himedialabs.com))
Preparation of HE Agar
- Measure the appropriate amount of HE agar powder (or the media components) and mix in the sterile distilled water of the required volume in a sterile conical flask (or glass bottle) according to the instruction of the manufacturing company (76.67 grams per 1000 mL in above media).
- Stir well using a sterile magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- DO NOT AUTOCLAVE THE MEDIUM.
- Cool the medium at around 40 – 45°C and dispense the molten medium in a sterile petri plate (around 25 mL in a 10 cm diameter petri plate) and allow it to cool and solidify at room temperature.
For performing the lead acetate paper test, we will be using Nutrient broth.
Composition of Nutrient Broth per 1000 mL
Peptone- 5.00 grams
Yeast Extract- 1.50 grams
Beef Extract (HM Peptone B)- 1.50 grams
Sodium Chloride- 5.00 grams
Final pH 7.4 ±0.2 at 25°C
(Reference: Nutrient Broth (himedialabs.com))
Preparation of Nutrient Broth
- Measure the appropriate amount of Nutrient broth powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company (13.00 grams per 1000 mL in the above media).
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components dissolve completely in water.
- Dispense about 5 mL of the medium in each test tube and loosely put on the cap or cotton plug the opening.
- Autoclave the tubes at 121°C and 15 lbs pressure for 15 minutes.
b. Reagents
Lead acetate paper (for lead acetate paper method)
c. Equipment
| Petri Plates Test tubes | Weighing Machine Inoculating wire | Bunsen burner Incubator | Inoculating loop Autoclave |
PPE and other general laboratory materials
d. Test Organism (Sample Bacteria)
Positive Control: Proteus mirabilis ATCC 29906
Negative Control: Shigella flexneri ATCC 12022
Procedure of Hydrogen Sulfide (H2S) Test
Tube Method for H2S Test
It is the most commonly followed method for the detection of H2S production.
- Using a sterile inoculating wire, touch a well-isolated colony from fresh culture (18 to 24 hours old) of the test bacterium.
- Inoculate the SIM medium tube by stabbing the medium more than halfway (up to 3 to 5 mm above the base of the tube) with the inoculating wire.
(While inoculating TSI, KIA, or other media with slant and butt, stab the butt and streak the slant surface using the inoculating wire.)
- Incubate the tube aerobically (with a loose cap) at 35±22°C for about 24 hours. (If suspected of Campylobacter, incubate for 72 hours.)
- Examine the formation of black-colored precipitate in the medium (turning medium to black color).
Plate Method for H2S Test
- Using a sterile inoculating touch a well-isolated colony from a fresh culture of the test bacterium.
- Streak culture over the agar plate to get well-isolated colonies.
- Incubate aerobically at 35±22°C for about 24 hours.
- Observe the color of the developed colonies.
Lead Acetate Paper Method for H2S Test
- Using a sterile inoculating touch a well-isolated colony from a fresh culture of the test bacterium and inoculate the nutrient broth tube.
- Place a lead acetate paper strip so that it hangs with one end just above the medium and the other end stuck at the neck of the tube by the screw cap or cotton plug.
- Incubate the tube aerobically at 35±22°C and observe for blackening of the paper strip after 24 hours. (For slow growers and Campylobacter, incubate for at least 3 days and observe for color change daily.)
Result and Interpretation of Hydrogen Sulfide (H2S) Test
Positive H2S Test
- Tube Method: Blackening of the culture media. (Whole media may turn black or the junction of slant and butt or any part of butt or slant may turn black. A black patch at any inoculated area is considered positive.)
- Plate Method: Black colonies and/or colorless or colored colonies with a black center.
- Lead Acetate Paper Method: Blackening (brownish-black color formation) of the lead acetate paper.
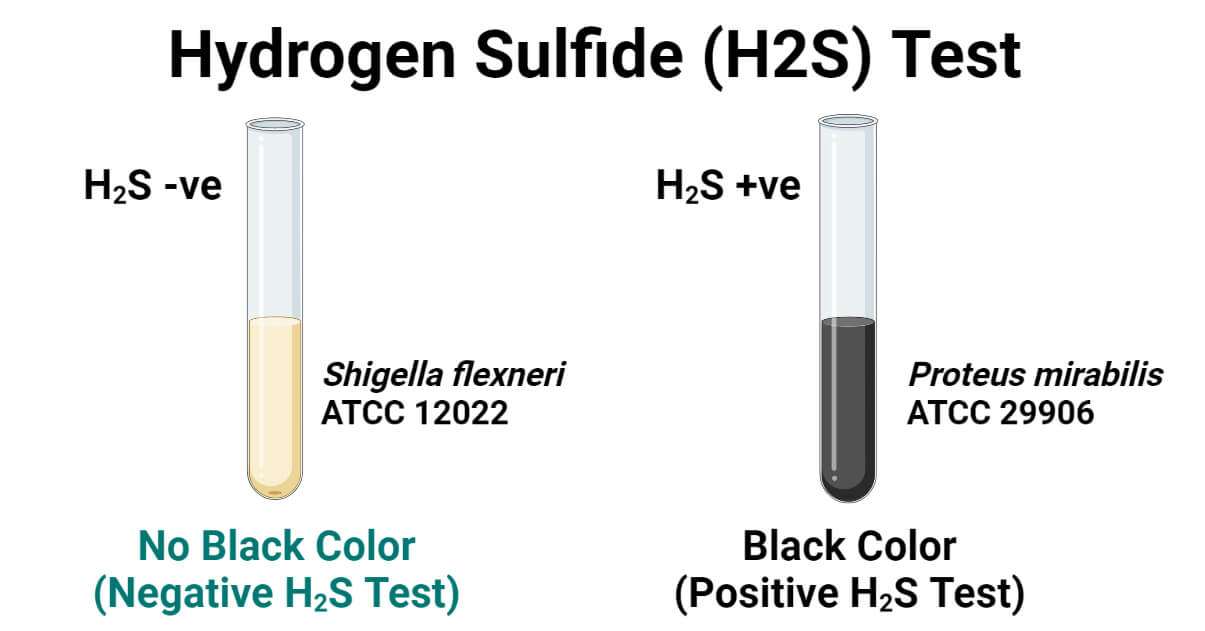
Negative H2S Test
- Tube Method: No blackening of media in any area.
- Plate Method: No black-colored colonies and/or no black-centered colonies.
- Lead Acetate Paper Method: No change in color of the lead acetate paper strip.
Quality Control
Proteus mirabilis ATCC 29906 will turn the medium/lead acetate paper black (and produce black colonies in the plate method).
Shigella flexneri ATCC 12022 will not turn/produce black color in the test tube, agar plate, and/or lead acetate paper.
H2S Test Positive Bacteria
Proteus spp., Citrobacter spp., Salmonella spp., Staphylococcus saprophyticus, Campylobacter spp., etc.
H2S Test Negative Bacteria
Klebsiella pneumoniae, Shigella spp., Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Vibrio cholerae, Yersinia pestis, etc.
Precautions
- Use the appropriate medium. Don’t use DCA, SS agar, and XLD medium if you want to test for Enterobacterales other than Salmonella and Shigella.
- Look for cracking of the medium in test tubes before inoculation. Don’t use it if there is a crack in the medium.
- Don’t use an inoculating loop for stabbing tubes.
- Do not put the cap of the test tubes tightly while incubating.
- Do not autoclave HE, DCA, and BS medium. (Look for instructions carefully before preparing the medium.)
- While preparing a medium that must not be autoclaved, always use sterile equipment and prepare in a sterile zone.
- Streak the plate with little inoculum and every streak you make must have some distance between them so that you can get well-isolated colonies.
- Lead acetate might inhibit bacterial growth, so don’t let the lead acetate paper strip touch the medium. Hang it slightly above the medium.
- If the test organism is a sucrose fermenter, don’t use a sucrose-containing medium.
Applications of Hydrogen Sulfide (H2S) Test
- For the presumptive identification of bacteria.
- Differentiation of Salmonella spp. (H2S test positive) from Shigella spp. (H2S test negative)
- Characterizing and identifying Enterobacterales
- For performing fecal culture and characterization of fecal pathogens
- Rapid fecal coliform detection in water.
- Separation of Lactobacilli (H2S test negative) from Erysipelothrix spp. (H2S test positive)
Limitations of Hydrogen Sulfide (H2S) Test
- It is not a confirmatory test; hence requires other biochemical test results for complete identification of the medium.
- Sucrose in the medium may inhibit H2S production.
- Some fastidious organisms may not grow in SIM, NB, DCA, and other basic mediums.
- Lead acetate is most sensitive in detecting H2S but is toxic to bacteria.
- Some may need incubation for long periods like 3 days for Campylobacter spp., and even more for other slow-growing bacteria.
- Confusion while choosing media for the test.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- Thakur, Swagata & Anokhe, Archana & Kalia, Vinay. (2021). Biochemical Test for Detecting Hydrogen Sulphide (H2S) Producing Bacteria. 2. 53-56. (PDF) Biochemical Test for Detecting Hydrogen Sulphide (H2S) Producing Bacteria (researchgate.net)
- Roser DJ, Ashbolt N, Ho G, Mathew K, Nair J, Ryken-Rapp D, Toze S. Hydrogen sulphide production tests and the detection of groundwater faecal contamination by septic seepage. Water Sci Technol. 2005;51(10):291-300. PMID: 16104433.
- Hydrogen sulfide (H2S)-producing bacteria (sulfate reducers, sulfite reducers, sulfur reducers, and other molecules with sulfur) – Qualitative and quantitative culture; Molecular identification (PCR and sequencing). – IVAMI
- Hydrogen Sulfide (H₂S) Production Test • Microbe Online
- Hydrogen sulfide (H2S) production test – Virtual Microbiology Lab Simulator Software (vumicro.com)
- Hydrogen Sulfide Test – Principle, Procedure, Uses and Interpretation (microbiologyinfo.com)
- Hydrogen Sulphide (H2S) Production Test – BiochemGems
- Hydrogen Sulfide Test – Procedure, Uses and Interpretation – Laboratoryinfo.com
- Hydrogen Sulfide (H2S) Test Principle, Procedure, Result (microbiologynote.com)
- Common Biochemical Tests in Microbiology: Hydrogen Sulphide Production Test – Labmonk
- Hydrogen Sulfide Test: Principle, Procedure, Uses and Interpretation (risingacademy.org)
- Hydrogen Sulfide Test: Principle, Procedure And Results Interpretation – BIOCHEMINSIDER

Alsalmo alikom can you please post the TSI for all Enterobacteriacea family.