Carbohydrates are a large group of organic compounds consisting of carbon, hydrogen, and oxygen which can be typically broken down into monomers to release energy in living beings.
- These are the most abundant biomolecules in the living body in terms of mass.
- Carbohydrates are also known as saccharides since many of those have a relatively small molecular weight with a sweet taste. This might, however, not be true for all carbohydrates.
- The empirical formula for carbohydrates is Cm(H2O)n, which holds for most monosaccharides. Carbohydrates are hydrates of carbon and are broadly defined as polyhydroxy aldehydes or ketones and their derivatives.
- These are widely distributed molecules in both plant and animal tissues serving as skeletal structures in plants and also in insects and crustaceans.
- They also occur as food reserves in the storage organs of plants and the liver and muscles of animals. They are an essential source of energy required for the various metabolic activities of living organisms.
- Plants are considerably more abundant in carbohydrates in comparison to animals.
- Other carbohydrate polymers lubricate skeletal joints and participate in recognition and adhesion between cells.
- Glucose, sucrose, cellulose, etc. are some of the most known and important carbohydrates found on earth.
What is Monomer?
- A monomer is the simplest molecule that forms the basic unit of polymers and thus is considered as the building blocks of polymers.
- Monomers bind with another monomer to form a chain of repeating molecules by the process of polymerization.
- Monomers can be either natural or synthetic in nature. Natural monomers are amino acids and monosaccharides, and examples of synthetic monomers are vinyl chloride and styrene.
What is a Polymer?
- A polymer is s substance consisting of large molecules that are composed of many repeating subunits or monomers.
- Polymers are formed by the binding of several monomeric units in the process termed polymerization.
- Polymers, like monomers, can be both synthetic and natural. Natural polymers include biomolecules like carbohydrates and proteins, whereas, synthetic polymers are polystyrene and polyvinyl chloride.
What is a Macromolecule?
- A macromolecule is a large molecule composed of thousands of atoms formed by the polymerization of smaller subunits called monomers.
- Macromolecules are composed of a large number of atoms that are bound together by covalent bonds. These macromolecules together form polymers.
- Macromolecules are also of two types; natural and synthetic. Natural macromolecules are biomolecules like carbohydrates and lipids, and synthetic macromolecules are synthetic polymers like plastics and rubber.
What are Covalent Bonds?
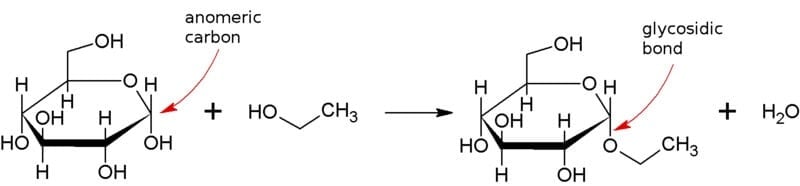
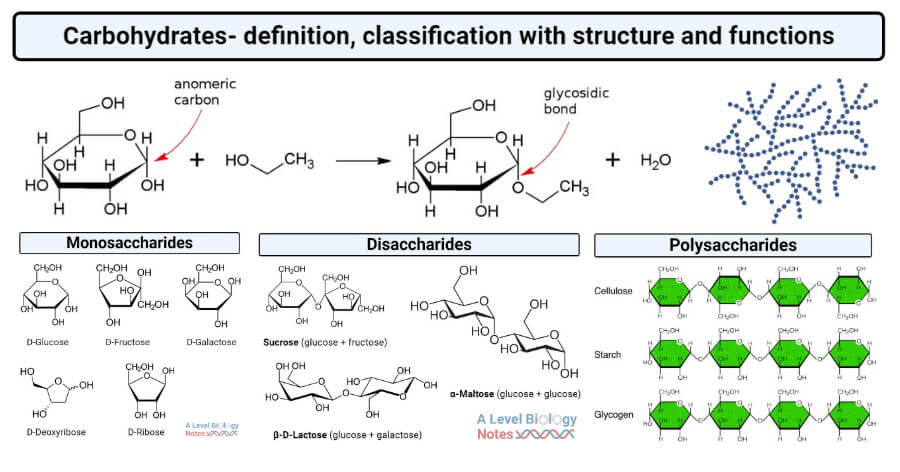
Figure: Formation of ethyl glucoside from glucose and ethanol, showing the anomeric carbon and the resulting glycosidic bond. Image Source: AxelBoldt.
- Monomeric units of carbohydrates are liked to one another by covalent bonds called glycosidic bonds.
- A glycosidic bond is formed between the carboxyl group present on the anomeric carbon of one monomer and the alcohol group present in another monomer.
- Covalent bonds in carbohydrates are special bonds that determine much of the shape of the more complex compound, mainly because these linkages inhibit the rotation of specific molecules toward each other.
- The covalent bonds in carbohydrates are either α or β-glycosidic linkages depending on the stereochemistry of the carbon atoms bound together.
- The linear chain in a carbohydrate molecule contains either an α-1,4-glycosidic bond or a β-1,4-glycosidic bond. The branching in carbohydrates, however, results due to a 1,6-glycosidic bond.
Interesting Science Videos
Reducing and non-reducing sugars
What are Reducing sugars?
- Reducing sugar is a sugar or a carbohydrate molecule with a free aldehyde group or a free ketone group which causes the molecule to act as a reducing agent.
- All monosaccharides, along with some disaccharides and polysaccharides, are reducing sugars.
- In the case of other polysaccharides and disaccharides, the aldehyde and ketone groups remain bound in the cyclic form.
- Most reducing sugars are sweet in taste. These sugars can be detected by tests like Benedict’s test and Fehling’s test as they give a positive result to these tests.
- Examples of reducing sugars include monosaccharides like galactose, glucose, glyceraldehyde, fructose, ribose, and xylose, disaccharides like cellobiose, lactose, and maltose, and polymers like glycogen.
What are Non-reducing sugars?
- A non-reducing sugar is a sugar or carbohydrate molecule that doesn’t have a free aldehyde or ketone group and thus cannot act as a reducing agent.
- Non-reducing sugars have aldehyde and ketone groups, but they are involved in the cyclic form of the sugar molecule.
- Some disaccharides and all polysaccharides are non-reducing sugars.
- Non-reducing sugars have a less sweet taste than the reducing sugars. These sugars can also be detected by tests like Benedict’s test and Fehling’s test as they give a negative result to these tests.
- Examples of non-reducing sugars include disaccharides like sucrose, maltose, and lactose and polysaccharides like starch and cellulose.
Formation of a glycosidic bond by condensation
- The process of formation of glycosidic bonds in carbohydrates is a condensation reaction which means that a molecule of water is formed during the process.
- The condensation reaction is formed between the OH group and the anomeric carbon of a sugar.
- These glycosidic bonds are formed in a dehydration synthesis reaction.
- When the alcohol attacks the anomeric carbon, the OH group of the carbon is replaced by the oxygen atom of the alcohol molecule. The OH group of the carbon and the remaining H atom of the alcohol are released in the form of a water molecule.
- The glycosylation reaction involves nucleophilic displacement at the anomeric center. As the reaction takes place at the secondary carbon atom by the attack of weak nucleophiles (sugar acceptors), it often follows a unimolecular SN1 mechanism.
- The result of a glycosidic bond is a sugar molecule bound to another molecule by an ether group.
- The ether bond formed has an oxygen atom bonded to two carbon atoms which result in a relatively stable structure than with the alcohol group. As a result, a glycosidic bond results in a more stable structure of the sugar.
Breakage of a glycosidic bond by hydrolysis
- The breakage of a glycosidic bond occurs by the process of hydrolysis by the addition of a water molecule.
- Hydrolysis of glycosidic bond occurs both in the presence of acid or an alkali.
- The OH group from the water molecule attacks the carbon atom involved in the glycosidic linkage.
- In acid-catalyzed hydrolysis, the hydrogen atom binds to the oxygen atom of the ether bond separating the monomeric units.
- In the case of polysaccharides, hydrolysis results in smaller polysaccharides or disaccharides, or monosaccharides.
- In the living system, hydrolysis of polysaccharides occurs in the presence of a group of enzymes termed hydrolases that catalyze the hydrolysis process.
Classification of Carbohydrates
Carbohydrates are classified into three different groups based on the degree of polymerization;
A. Monosaccharides
- Monosaccharides are the simplest form of sugar that cannot be hydrolyzed into smaller units.
- The monosaccharides, often called simple sugars, are compounds that possess a free aldehyde (—CHO) or ketone (= CO) group and two or more hydroxyl (—OH) groups.
- Monosaccharides are considered as fuel molecules that are involved in the formation of polymers like polysaccharides and nucleic acids.
- Monosaccharides are further divided into different groups based on various characteristics like the placement of the carbonyl group, the number of carbon atoms it contains, and the stereochemistry of the carbon atom.
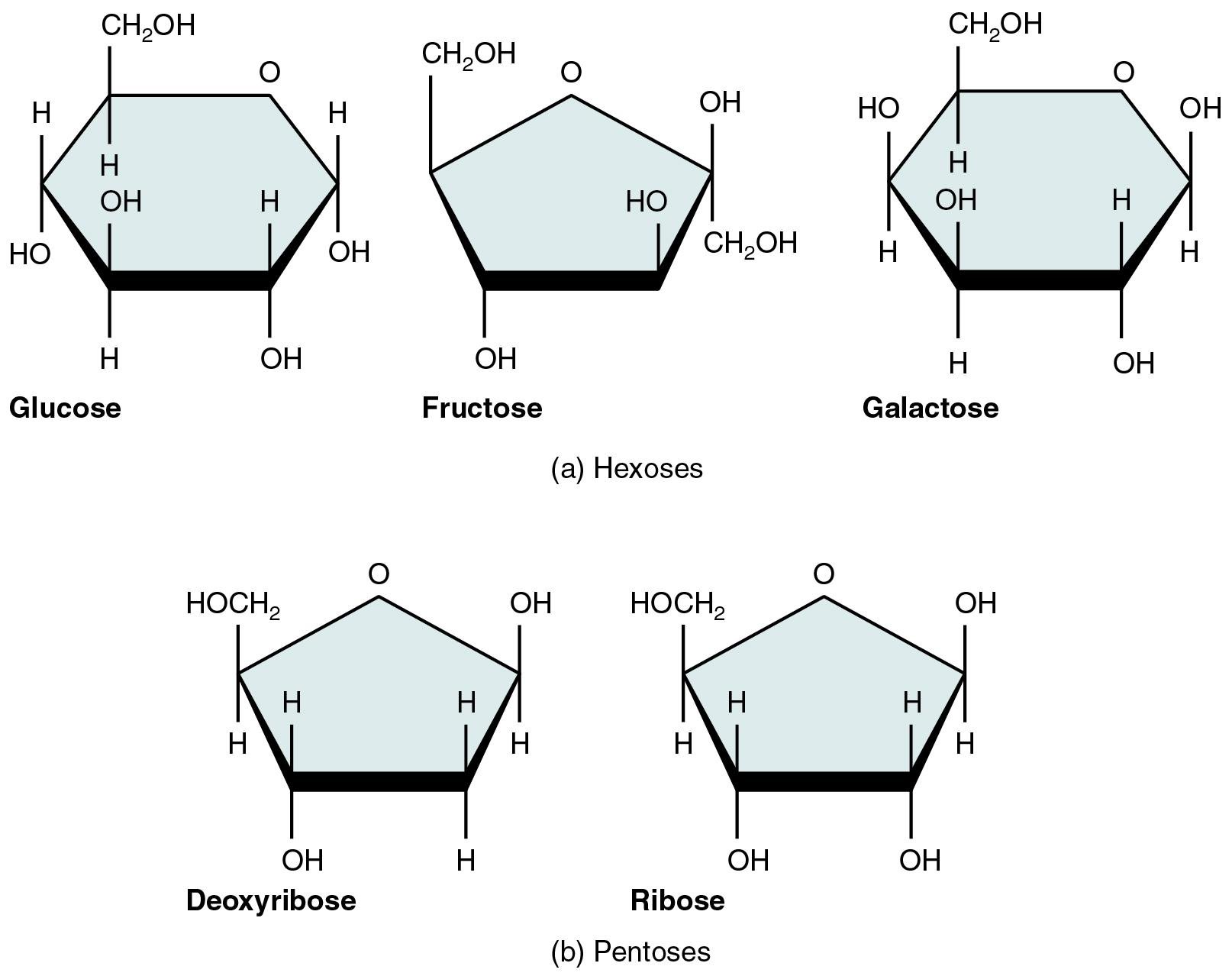
Figure: Some examples of Monosaccharides.
Based on the placement of the carbonyl group, monosaccharides are of two types;
Aldoses
- Monosaccharides having an aldehyde group as the carbonyl group are termed as aldoses.
Ketoses
- Monosaccharides having a ketone group as the carbonyl group are termed as ketoses.
- Based on the number of carbon atoms, monosaccharides are divided into trioses, tetroses, pentoses, hexoses, and heptoses having three, four, five, six, and seven carbon atoms.
- Monosaccharides are further divided into D and L forms based on the orientation of the asymmetric carbon furthest from the carbonyl group.
Structure
- The empirical formula of all monosaccharides is (CH2)O which indicates that the central carbon atom bonded to two hydrogen atoms and an oxygen atom.
- A carbonyl group is present in all monosaccharides where if the group is present at the end, it forms an aldose and if it is present in the middle, it forms a ketose sugar.
- Monosaccharides with more than five carbon atoms exist in the form of rings in the solution state.
- The ring structure is formed when the hydroxyl group on the fifth carbon reacts with the first carbon atom.
- Monosaccharides are composed of a single molecule of sugar with no glycosidic bond.
- All monosaccharides are reducing sugars with a free aldehyde or ketone group.
Functions
- Monosaccharides are one of the main fuels for energy in living beings with most of them providing 4kcal energy per gram of the carbohydrate.
- Monosaccharides are involved in the synthesis of various biomolecules like ribose and ribulose involved in the synthesis of nucleic acids, coenzymes like NAD, NADH, Coenzyme A, etc.
- Several monosaccharides linked together by glycosidic bonds result in the process of polymerization, forming larger polysaccharides.
- In plants, ribulose biphosphate acts as the carbon dioxide acceptor during photosynthesis.
Example of Monosaccharide
Glucose
- Glucose is an important monosaccharide that provides energy and structure to different parts of a cell.
- Glucose is a six-carbon compound with the molecular formula C6H12O6. It is an aldohexose with six carbons and a free aldehyde group.
- The glucose molecule exists in both open-chain and ring form, the latter formed as a result of intermolecular interaction between the aldehyde carbon and the C-5 hydroxyl group.
- Glucose exists in two forms; α-glucose and β-glucose. If the -OH group attached to the anomeric carbon is below the ring, the molecule is alpha glucose, and if the -OH group is above the ring, the molecule is beta glucose.
- The α-glucose forms the tasty part of the plants like fruits and flowers, whereas, the β-glucose forms the structurally hard part of the plant like the stem and roots.
- These two forms, however, can interconvert as the glucose changes structure from open-chain to cyclic or ring form.
- Glucose is an essential monosaccharide that is broken down during glycolysis providing energy and precursors for cellular respiration.
- Long chains of glucose molecules are linked together by glycosidic bonds to form essential polysaccharides like starch and glycogen.
- Glucose, together with other molecules, also forms the basis of various structural parts of a cell.
B. Disaccharides
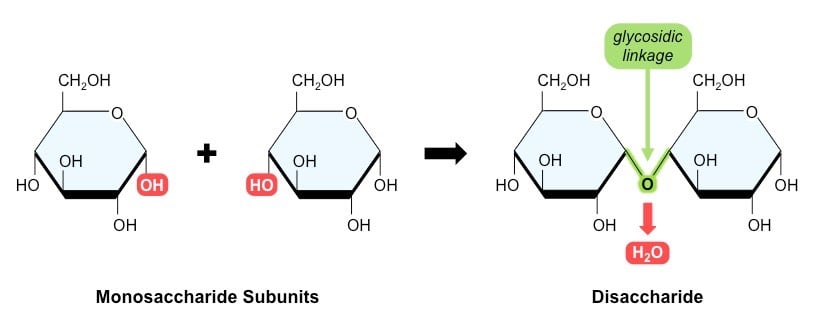
Figure: Some Examples of Disaccharides.
- A disaccharide is a sugar molecule composed of two monomeric units linked together by a glycosidic bond, resulting from a condensation reaction.
- Disaccharides are the simplest polysaccharides composed of either identical or two different monosaccharides.
- The most common and unmodified disaccharides have the molecular formula C12H22O11.
- Disaccharides are of two types; reducing and non-reducing disaccharides. Reducing disaccharides have a free carbonyl group, whereas non-reducing disaccharides do not have a free carbonyl group.
- Disaccharides like maltose, sucrose, and lactose have the same molecular formula but have different atomic arrangements.
- Disaccharides are an important source of energy as they can be broken down to produce monosaccharides that are involved in the metabolic pathways within living beings.
C. Polysaccharides
- Polysaccharides are sugar molecules with more than ten monosaccharide units bonded together by glycosidic bonds.
- Polysaccharides are also termed glycan.
- Polysaccharides are a long chain of monosaccharides where the polysaccharide can be either homopolysaccharide or heteropolysaccharide.
- Homopolysaccharides are formed of identical monosaccharides, and heteropolysaccharides are formed of different monosaccharides.
- Polysaccharides have different forms depending on the monosaccharides present and the carbon atoms connected to one another.
- Some polysaccharides are linear, while others are branched.
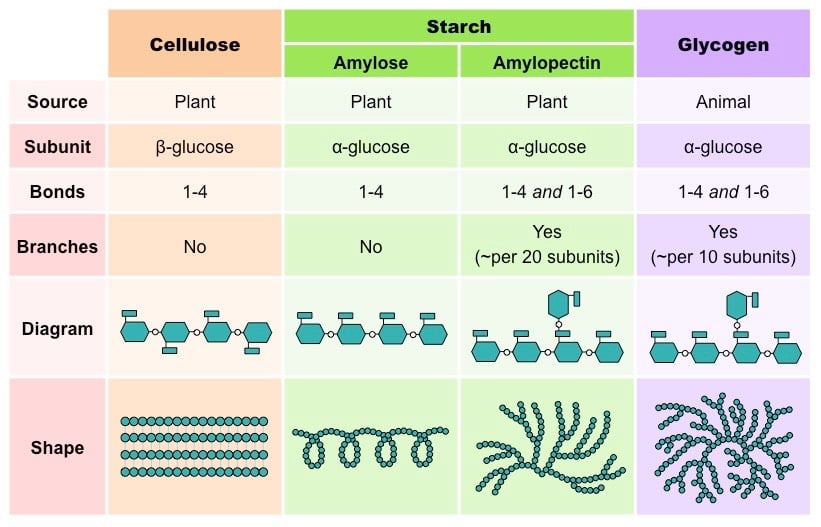
Figure: Some examples of Polysaccharides.
Examples of Polysaccharides
1. Starch
Starch is a polysaccharide comprising glucose monomers joined together by glycosidic linkages. Starch is an organic compound found in all living plants which is manufactured from the excess glucose produced during photosynthesis. Starch is the form of reserve food in plants stored in chloroplasts in the form of granules and storage organs like roots, tuber, stem, and seeds.
Structure
- Starch is a homoglycan composed of a single type of sugar unit, regardless of the source of the starch.
- A single starch molecule has 300 to 1000 glucose units bound together.
- Most starches are composed of two kinds of polysaccharides, a linear α-(1→4) linked glucan, called amylose, and an α-(1→4) linked glucan with 4.2 to 5.9% α-(1→6) branch linkages, called amylopectin.
- The ratio of amylose to amylopectin also varies, depending on the source of the starch; it ranges from 17 to 70% amylose and a corresponding 83 to 30% amylopectin.
- α-amylose or simply amylose has a molecular weight range of 10,000 to 50,000, which may be formed in plant cells by the elimination of a molecule of water from a glycosidic OH group of one α-D-glucose molecule and alcoholic OH group on carbon 4 of the adjacent α-D-glucose molecule.
- The linkage in amylose is, thus, an α-1, 4-glucoside.
- β-amylose or amylopectin has a high molecular weight range of 50,000 to 1,000,000, thus indicating the presence of 300–5,500 glucose units per molecule.
- Additional α-1,6-glucoside linkages are found in amylopectin in addition to the α-1,4-glucoside linkages.
- In plants, starch molecules are arranged in the form of semi-crystalline granules.
Functions
- Starch is the most common and essential storage form of carbohydrates in plants.
- It is a major source of energy in a carbohydrate diet where the hydrolysis of starch yields glucose which is further metabolized to produce energy.
2. Glycogen
Glycogen is a branched polysaccharide that is a major form of glucose in animals and humans. It is often termed as ‘animal starch’ and is stored in the liver and muscles of animals.
Structure
- Glycogen is a branched-chain polysaccharide and resembles amylopectin in its structure.
- Glycogen molecule is composed of glucose subunits that are linked together by α-1,4 linkages that branch off via α-1,6 linkages every ten glucose residues.
- These linkages result in a helical polymer structure that exists in the form of granules in the cytoplasm.
- Glycogen is similar to starch but has more branches and is more compact than starch.
- Glycogen is synthesized in the body when there is an excess of glucose produced in the body.
Functions
- The primary function of glycogen is the storage of excess glucose in the body when the blood glucose level increases.
- Glycogen then breaks down into glucose molecules to provide energy to the body when the blood glucose level decreases.
- By allowing the formation and hydrolysis, glycogen helps to maintain the blood glucose level.
- About 6-10% of the weight of the liver is made up of glycogen which is converted into glucose molecules during fasting.
- Reserved glycogen in the muscle cells serves as a fuel or the supply of ATP during muscle contraction.
3. Cellulose
Cellulose is the most abundant extracellular structural polysaccharide in plants and the most abundant of all biomolecules in the biosphere. Cellulose is found in all land plants but is absent in meat, egg, fish, and milk. It, however, cannot be metabolized by the human system. Cellulose occurs in the cell walls of plants where it contributes in a major way to the structure of the organism.
Structure
- The molecular weight of cellulose ranges between 200,000 and 2,000,000, thus corresponding to 1,250–12,500 glucose residues per molecule.
- It is formed by the glycosidic linkage between the OH group on C1 of one β-D-glucose molecule and the alcoholic OH group on C4 of the adjacent β-D-glucose molecule.
- It resembles in structure with amylose except that the glucose units are linked together by β-1, 4-glucoside linkages.
Functions
- Cellulose is the major structural polysaccharide in plants that forms the various structure of plant cells, including the cell wall.
- Cellulose has high rigidity and strength that enables the cell to have a solid structure and shape.
- Cellulose can be degraded into smaller monosaccharides like glucose which can then be broken down metabolically to produce energy.
- It is also important for the formation of paper and wood.
References
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company
- Jain JL, Jain S and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Robyt J. (2008) Starch: Structure, Properties, Chemistry, and Enzymology. In: Fraser-Reid B.O., Tatsuta K., Thiem J. (eds) Glycoscience. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-30429-6_35
