Chromatography is a method used to separate the individual components of a mixture on the basis of their interactions with a stationary and a mobile phase. Each component interacts differently with the two phases which causes them to move at different speeds and separate.
- It was first developed by Russian botanist Mikhail Tswett in 1906 when experimenting with plant pigments.
- Tswett poured a plant pigment solution on top of a column with an adsorptive material and added a solvent to wash it through the column. As the solution moved down, the pigments separated into bands of different colors.
- Over time, chromatography has advanced to different types which allow separation from small molecules to complex biomolecules.
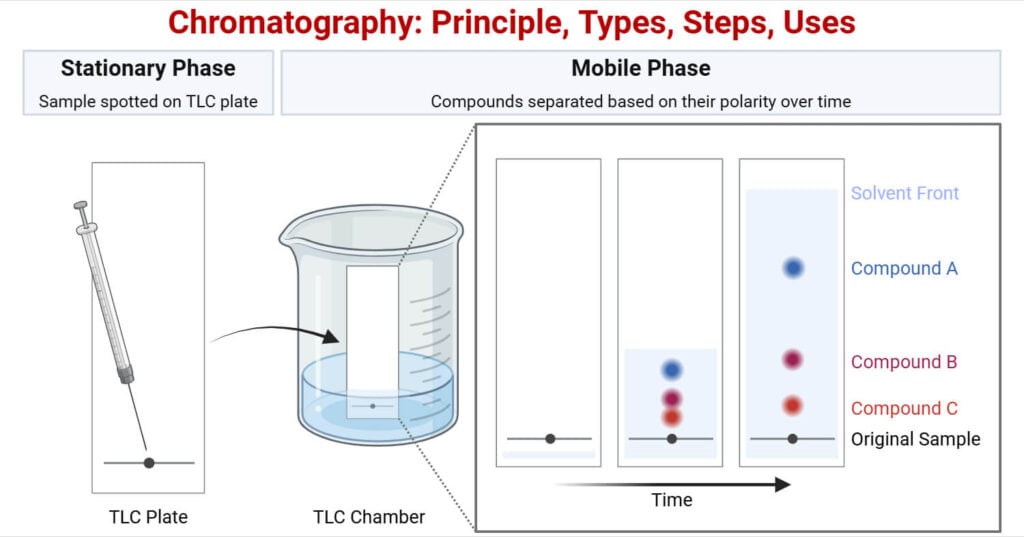
Principle of Chromatography
The main principle of chromatography is that different substances interact differently with the stationary and mobile phases and this difference in interaction separates the compounds. As the mobile phase moves with the sample through the fixed stationary phase, each compound moves at a different speed based on its properties like size, charge, or affinity to the stationary phase resulting in separation. The separated components are represented as peaks on a chromatogram and identified by its retention time which is the time a compound takes to move through the system.
Components and Parts of Chromatography
a. Sample or Analyte
The sample or analyte is the mixture that needs to be separated. Different components of the sample behave differently and are separated based on how they interact with the mobile and stationary phases.
b. Stationary Phase
This is the fixed material that can be solid or liquid coated on a surface. As the analyte passes through it, the stationary phase holds or slows down certain molecules based on their properties. Some of the commonly used stationary phases are filter paper, silica, and beads.
c. Mobile Phase
This is the moving fluid or solvent that carries the mixture and flows through the stationary phase. The mobile phase can be a liquid or a gas. Some of the commonly used mobile phases are water, acetic acid, or gases like hydrogen and nitrogen.
d. Chromatography Column
It is the container that holds the stationary phase where separation occurs. It is used in column-based methods like gas chromatography and HPLC. Chromatography columns are usually cylindrical in shape. The size and material can vary depending on the type of chromatography.
e. Detector
A detector is used at the end of the system to identify and measure the individual components as they separate. It shows results as peaks on a graph called a chromatogram. Different types of detectors are used like UV light, mass spectrometry, or flame ionization.
Types of Chromatography
Some of the types of chromatography are:
- Adsorption Chromatography
- Affinity chromatography
- Anion exchange chromatography
- Capillary Electrophoresis Chromatography
- Cation exchange chromatography
- Chiral Chromatography
- Column chromatography
- Countercurrent Chromatography
- Dye ligand chromatography
- Fast Protein Liquid Chromatography
- Flash chromatography
- Gas chromatography (GS)
- Gas Chromatography-Mass Spectrometry (GC-MS)
- Gas Liquid Chromatography
- Gel-permeation (molecular sieve) chromatography
- High performance liquid chromatography (HPLC)
- Hydrodynamic Chromatography
- Hydrophobic interaction chromatography
- Ion Chromatography
- Ion exchange chromatography
- Liquid chromatography
- Multimodal or Mixed-Mode Chromatography (MMC)
- Paper chromatography
- Partition Chromatography
- Pseudoaffinity chromatography
- Reverse-phase chromatography
- Size Exclusion Chromatography
- Supercritical Fluid Chromatography (SFC)
- Thin-layer chromatography
- Two-Dimensional Chromatography
Chromatographic methods can be grouped into different categories based on different factors. Some to these categories are:
Based on the geometry of the system
Two main types based on the geometry or the shape of the chromatographic setup: Planar and Column Chromatography.
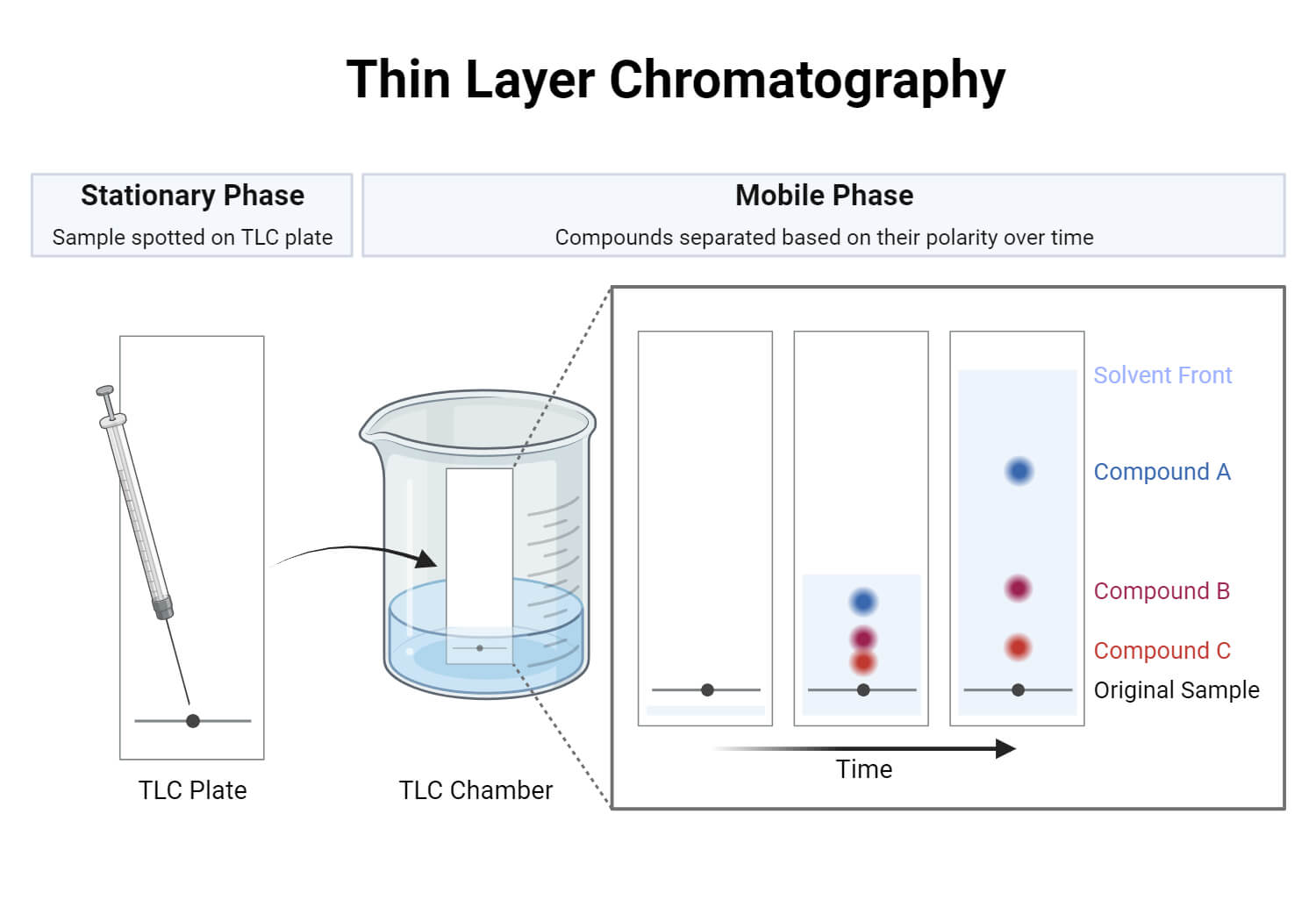
- Planar Chromatography: In this type, the stationary phase is spread out on a flat surface. The mobile phase moves across the surface by capillary action. Examples: Paper Chromatography and TLC.
- Paper Chromatography uses filter paper as a stationary phase where samples are separated. This is suitable for separating plant pigments, dyes, and amino acids. In this method, a small spot of the sample is placed near the base of a filter paper which is placed vertically in a sealed container with solvent just below the sample spot. The solvent moves up the paper along with the sample due to capillary action and separates the sample components based on their affinity for the solvent and the paper.
- Thin Layer Chromatography (TLC) uses a thin layer of solid particles like silica gel or alumina as the stationary phase. This thin layer of adsorbent material is coated on a flat plate where separation occurs. It works similarly to paper chromatography but has better separation and faster results. TLC is used mainly for separating and identifying organic compounds.
- Column Chromatography: Here, the stationary phase is packed inside a vertical column and compounds are separated as they move with the solvent at different rates through the column. Examples: Gas chromatography and HPLC.

Based on the retention mechanism
Chromatographic methods can also be categorized based on the mechanism of separation or on how the substances are retained by the stationary phase.
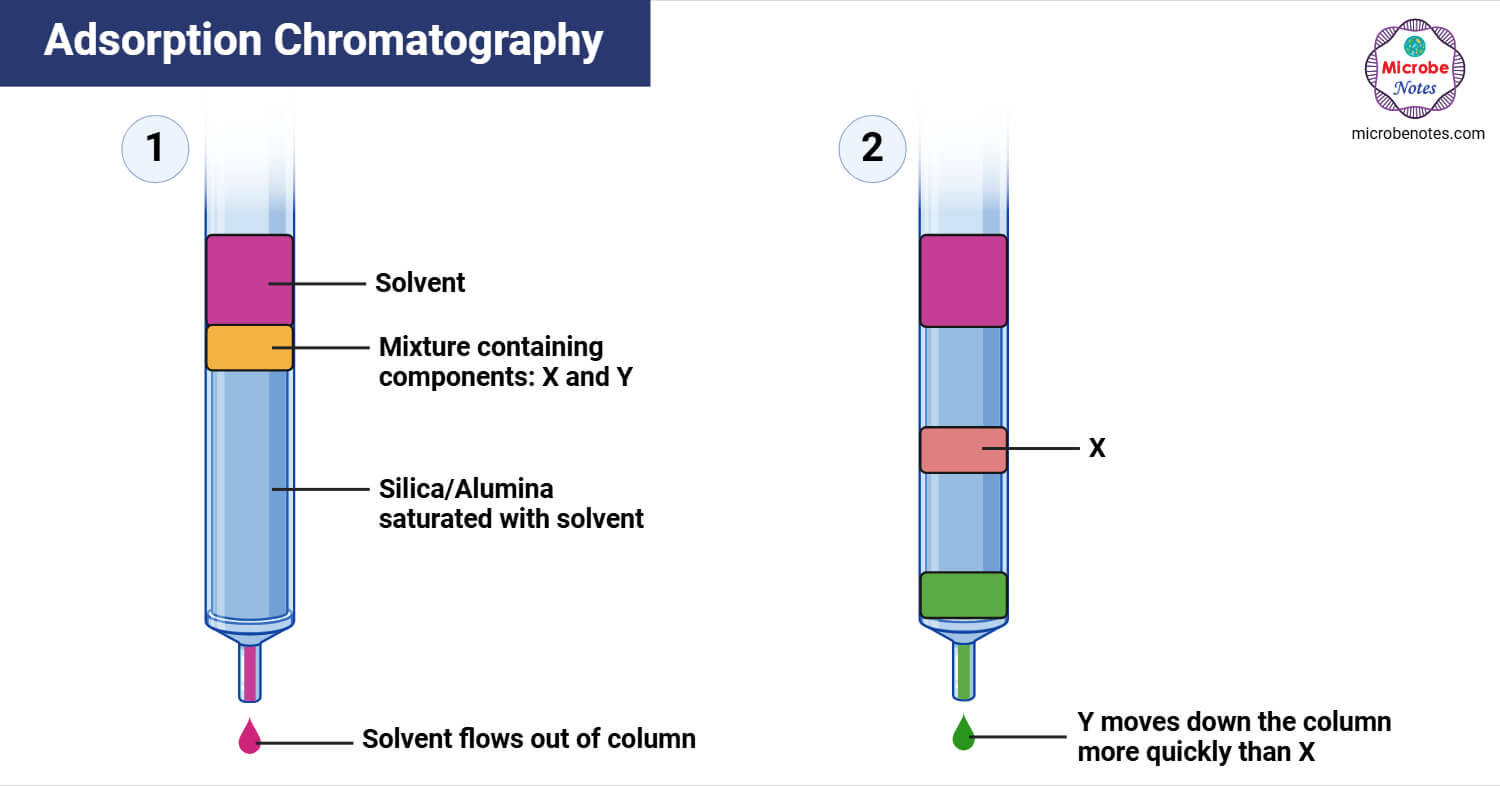
- Adsorption Chromatography separates compounds based on how strongly they are adsorbed to the stationary phase. Components with weaker adsorption to the stationary phase move faster and those with stronger binding move slower.
- Partition Chromatography separates compounds based on their solubility properties or differential partitioning between the liquid mobile phase and stationary phase. This is measured by the partition coefficient which is the ratio of how much a substance prefers one phase over the other. Compounds that are more soluble in the stationary phase move slowly while those more soluble in the mobile phase move more quickly.
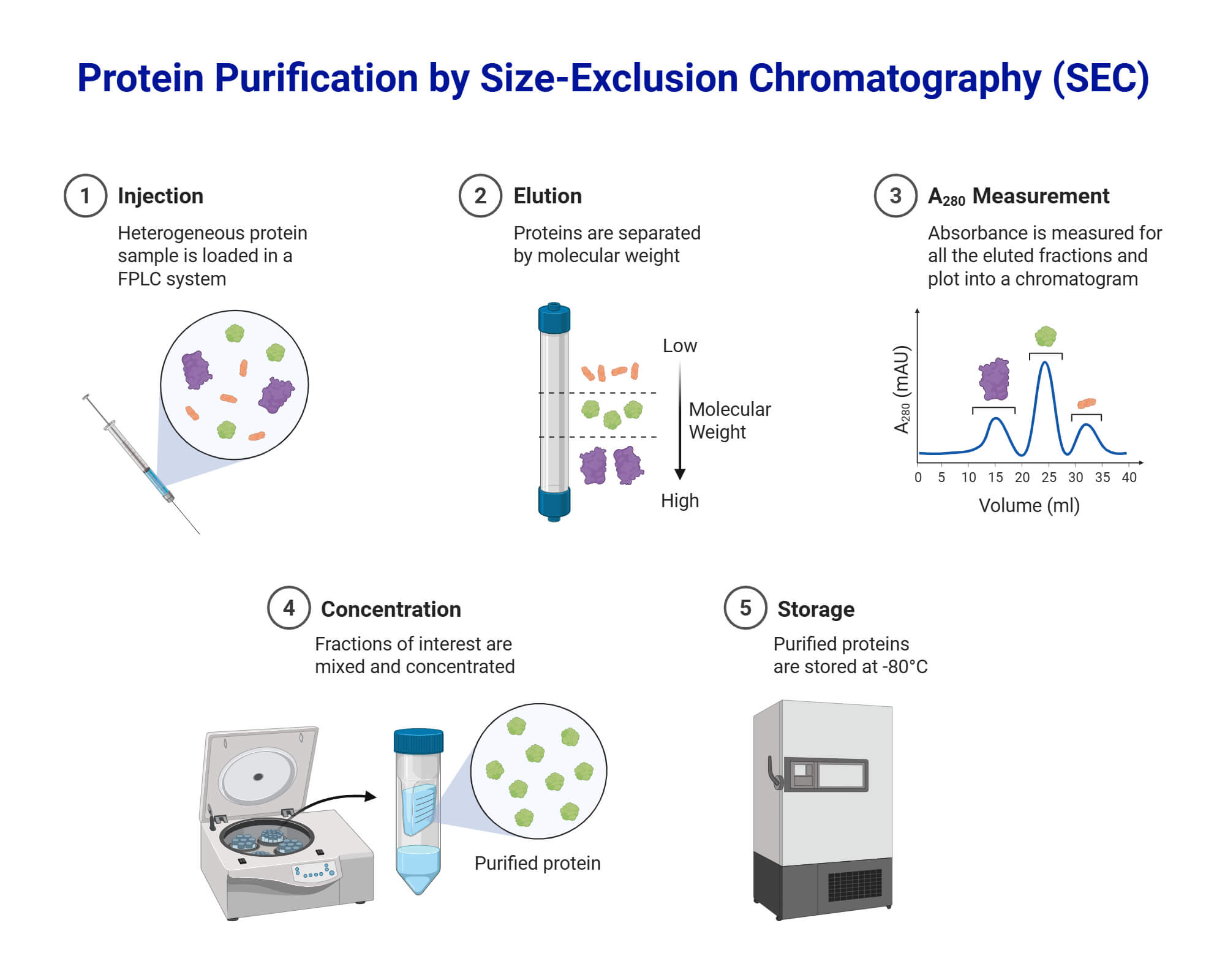
- Size-Exclusion Chromatography (SEC) separates compounds based on their molecular size. It is commonly used for large molecules like proteins. It is also called gel filtration or gel permeation chromatography. The stationary phase contains porous materials. As the solution moves through porous materials, smaller molecules will enter the pores and move slowly while larger molecules can pass through the column and elute faster.
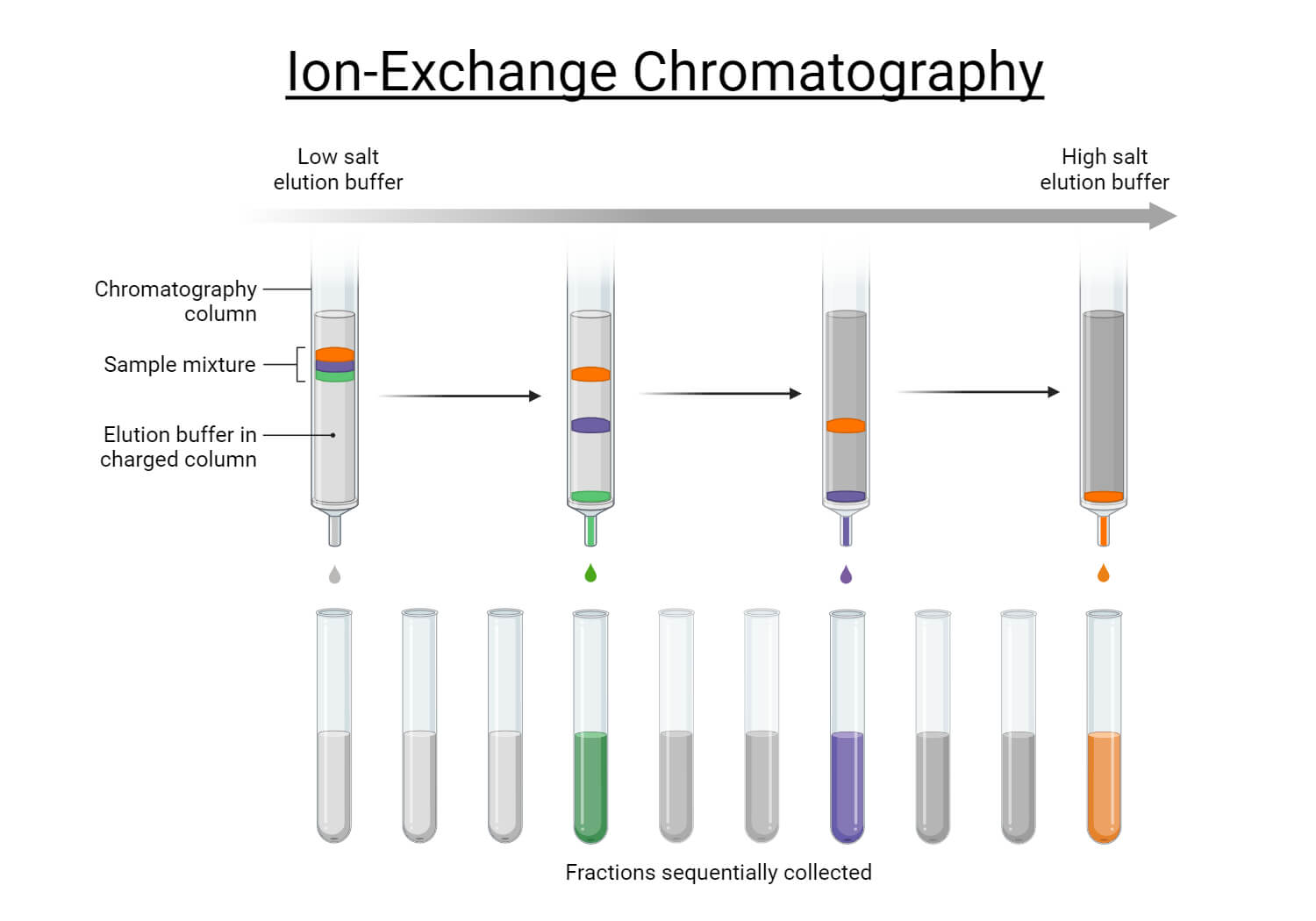
- Ion Exchange Chromatography (IEC) is used to separate molecules by their net charge. This method separates charged ions or biomolecules. The stationary phase has charged resins that attract oppositely charged molecule in the sample. The two types of IEC are:
- Cation exchange chromatography uses negatively charged resins in stationary phase to retain positively charged molecules.
- Anion exchange chromatography uses positively charged resins in stationary phase to retain negatively charged molecules.
- Affinity Chromatography uses specific biological interactions like antibody and antigen or enzyme and substrate binding to isolate target molecules. A ligand is attached to the stationary phase that specifically bind the target molecule. It is used for isolating proteins, enzymes, antibodies, receptors, and hormones.

Based on the phases involved
This refers to the physical states of the phases.
- Gas Chromatography (GC) separates volatile compounds. In GC, the mobile phase is a gas, also called the carrier gas which is usually helium, hydrogen, or nitrogen. Depending on the type of stationary phase used, the two types of GC are: gas-solid chromatography and gas-liquid chromatography. It can be used in detecting pollutants in air or analyzing perfumes.
- Liquid Chromatography (LC) uses liquid as the mobile phase. It can also be divided into liquid-solid chromatography and liquid-liquid chromatography. High-Performance Liquid Chromatography (HPLC) is an advanced method of LC that uses high pressure and specialized columns for better resolution and speed.
Procedure or Steps of Chromatography
a. Sample Preparation
The sample is first processed using methods like solid-phase extraction, liquid-liquid extraction, or protein precipitation. Then, filtration and centrifugation are done to remove any contaminants. The preparation method is different depending on the chromatographic method used.
b. Phase selection
Both stationary and mobile phases must be carefully selected based on the properties of the sample and the separation method. The stationary phase stays fixed inside the system and can be a solid or liquid. It is packed into a column or coated onto a surface like paper or plate. The mobile phase acts as the carrier to move the sample through the system.
c. Sample Application
The prepared sample is introduced into the chromatographic system. In methods like TLC or paper chromatography, the sample is spotted near the bottom of the plate or strip. In column chromatography like HPLC, the sample is injected into the system.
d. Separation
As mobile phase moves through stationary phase, it carries the components of the sample at different rates. Some components move quickly with the mobile phase while others are slowed by the stationary phase. This separates the components of the sample. In some methods, additional solvent extraction is required to collect analytes for further analysis.
e. Detection and Analysis
After separation, detectors are used to visualize the separated components. Some common detection methods are UV spectroscopy, staining reagents, refractive index detection, flame ionization detection, and mass spectrometry. Detectors generate voltage signals recorded as chromatograms which are analyzed to identify and quantify the analytes. Each peak in the chromatogram represents a specific analyte and the peak area or height indicates its concentration. The retention time is used to identify the compound.
Factors Affecting Chromatography
- Sample Properties: The molecular properties of the analyte including attributes like the size, shape, charge, hydrophobicity, and hydrogen bonding can affect the chromatographic process.
- Mobile Phase: Properties of the mobile phase like the solvent composition, polarity, pH, and operational parameters like temperature can influence the separation process.
- Stationary Phase: The properties of the stationary phase are also important factors that affect chromatographic separation. Its chemical composition, particle size, porosity, and surface chemistry can affect how analytes interact and separate.
- Temperature: It is an important factor that affects the properties of the mobile phase and interactions with the stationary phase. Higher temperature increases the diffusion of analytes. It also reduces the viscosity of the mobile phase which helps in smoother flow and increases the efficiency of the separation process.
- Flow rate: The flow rate of the mobile phase also affects the chromatography process. Higher flow rates allow analytes to pass through the column more quickly and result in shorter analysis time. However, better separation is achieved with lower flow rates.
Common Products and Manufacturers of Chromatography
Chromatography products include instruments, columns, consumables, and software from different manufacturers. Some of the common ones are:
| Manufacturer | Common Products |
| Agilent Technologies | InfinityLab LC systems, 8890 GC system, ZORBAX HPLC columns, DB-5 GC columns, OpenLab CDS |
| Waters Corporation | ACQUITY UPLC systems and columns, Alliance HPLC, Atlantis columns, Empower |
| Thermo Fisher Scientific | Vanquish HPLC and UHPLC systems, TRACE 1600 GC series, Chromeleon |
| Shimadzu | Nexera HPLC systems, Nexis GC-2030, Shim-pack columns, LabSolutions CS |
| Merck | Chromolith HPLC columns, Supelco GC columns, Supelclean SPE kits |
| Phenomenex | Kinetex and Luna LC columns, Zebron GC columns, Strata SPE products |
| Restek | Rxi GC columns, Resprep SPE products |
| PerkinElmer | Elite GC columns, Flexar HPLC systems, TotalChrom |
| GL Sciences | Inertsil HPLC columns, InertCap GC columns |
| Cytiva | HiTrap columns, UNICORN |
| Bio-Rad Laboratories | BioLogic chromatography systems, ENrich columns, Aminex HPLC columns |
Applications of Chromatography
- Chromatography can be used in environmental testing to monitor air and water quality.
- It has applications in forensic science to detect toxic substances in the body and analyze samples like blood, urine, or saliva.
- It can be used in bioprocessing or product purification like monoclonal antibodies and vaccines.
- It has applications in the food industry to check the safety and quality of food products. It helps to detect contaminants and additives in food products. It can also determine the ingredient composition which is used for nutritional assessment and accurate labeling.
- It has applications in the chemical industry to check the purity and determine the chemical properties of different compounds.
- It has applications in the pharmaceutical industry to develop drugs and check the quality of the products.
Advantages of Chromatography
- It can separate all the component of a mixture with little to no prior information about the sample composition.
- It is a versatile method as it is compatible with a wide range of substances.
- It is accurate and can handle complex mixtures.
- It is highly sensitive and can detect substances in extremely small amounts.
- It can be easily combined with additional detectors like mass spectrometry.
- It can be applied to different molecules from the smallest gases to large biological macromolecules.
Limitations of Chromatography
- Chromatography requires expensive instruments and consumables. Setting up chromatographic systems like HPLC or GC require high initial investment. Operational expenses like regular maintenance and consumables are also high.
- Preparing samples for chromatography is complex.
- It may face difficulties when separating compounds with similar properties.
- It requires skilled personnel with specialized training and expertise to operate chromatography instruments, interpret results, and troubleshoot.
Troubleshooting and Safety Considerations
- Baseline drift or background noise can occur due to temperature changes and contamination. This can be resolved by keeping the temperature stable, cleaning the system, or replacing the detector.
- Sample overloading, wrong pH, or unsuitable sample solvents can cause poor peak shape which can be fixed by adjusting sample volume, optimizing pH, and replacing the column if needed.
- Overlapping peaks can occur due to poor separation or by using unsuitable column. The right column and separation conditions must be used.
- When working with toxic or flammable chemicals, safety measures should be adopted like using fume hoods and personal protective equipment (PPE). Chemicals should be properly labeled and stored.
- HPLC or GC systems should be regularly checked for pressure leaks or blockages. Pressure should be safely released before opening any pressurized components.
- Laboratories must maintain updated standard operating procedures (SOPs).
- Waste including solvents, buffers, and contaminated materials must be properly disposed. Labeled waste containers must be used and incompatible chemicals should not be mixed.
Recent Advances and Innovations in Chromatography
- Compact and portable chromatographic devices using microfluidics systems are being developed which allow onsite analysis and reduces sample consumption.
- Innovations in stationary phase materials such as development of monolithic columns and nanoparticle-based phases.
- Chromatographic methods are combined with other analytical methods like Mass Spectrometry that improves separation.
- Artificial intelligence (AI) can be used to predict retention, analyze complex data, and improve accuracy.
- Sustainable practices are being adopted such as minimizing wastes and using natural solvents.
Conclusion
Chromatography is a useful method for separating and studying different parts of a mixture. The basic idea of all chromatographic methods is that different substances move at different speeds through a material so they can be separated and analyzed. It has different applications including chemical analysis, forensic investigations, environmental studies, pharmaceutical testing, and food safety. With new advances and innovations, chromatographic methods are becoming more accurate and efficient.
References
- Chromatography Troubleshooting Guides | Thermo Fisher Scientific – NP. (n.d.). Retrieved from https://www.thermofisher.com/np/en/home/industrial/chromatography/chromatography-learning-center/chromatography-consumables-resources/chromatography-troubleshooting-guides.html
- Coskun O. (2016). Separation techniques: Chromatography. Northern clinics of Istanbul, 3(2), 156–160. https://doi.org/10.14744/nci.2016.32757
- Debnath, S., Das, M., Mondal, S., Sarkar, B. K., & Babu, G. (2025). Advances in chromatography: contemporary techniques and applications. Essential Chem, 2(1), 1–27. https://doi.org/10.1080/28378083.2025.2466624
- Factors affecting chromatographic separation | Solubility of things. (n.d.). Retrieved from https://www.solubilityofthings.com/factors-affecting-chromatographic-separation
- Future Trends in Chromatography | Solubility of Things. (n.d.). Retrieved from https://www.solubilityofthings.com/future-trends-chromatography
- Keller, A, R., Giddings, & Calvin, J. (2025, April 8). Chromatography | Definition, Types, & Facts. Retrieved from https://www.britannica.com/science/chromatography/Subsequent-developments
- Kumar, P. (2015, October 16). Top 12 types of chromatographic techniques | Biochemistry. Retrieved from https://www.biologydiscussion.com/biochemistry/chromatography-techniques/top-12-types-of-chromatographic-techniques-biochemistry/12730
- Pan, S. (2025, February 9). Chromatography – principle, types, applications – Biology Notes online. Retrieved from https://biologynotesonline.com/chromatography-principle-types-applications/
- Patrick, G. (2023, June 22). Top 10 chromatography instrument companies opening new dimensions of chemistry. Retrieved from https://www.verifiedmarketresearch.com/blog/top-chromatography-instrument-companies/
- Premnath, S. M., & Zubair, M. (2024, January 11). Chromatography. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK599545/s
- Safety Considerations in Chromatography | Solubility of things. (n.d.). Retrieved from https://www.solubilityofthings.com/safety-considerations-chromatography

Thank you for the wonderful article
perfect
OMG 30 DIFFERENTS TYPES OF CHROMATOGRAPHY . THIS ARTICLE IS SO EDUCATIVE THNK YOU MISS
Effective article
Biochemistry
What is the meaning of chromatography
chromo means is colour and graphy means is measur the colour
It is the seperation and purification technique that separates components of a mixture base on their differing affinities for a stationary phase and a mobile phase which are packed into a column
Thanks so much for the simplification of Principle of Chromatography
Excellent!
Very helpful, thanks so much 8
Because mobile phase travels against gravity so the gravitational force on it causes it to slow down
Osm article …. Thanks
Thanks for this article…. It was excellent..,..
Thanks you
Thank You So Much. Needed Info For A Project And Got It All Here!
Very good , i like it very much
Why is the rate of movement of mobile phase less in ascending Paper Chromatography?
It is because of the molecular weight. Components with large molecular weight rise up slowly.
Excellent article really like it
excellence article related to chromatography.brief revision of chromatography. thanks all team.
Excellent article.
Why is the rate of movement of mobile phase less in ascending Paper Chromatography?
What is Chromatogram?