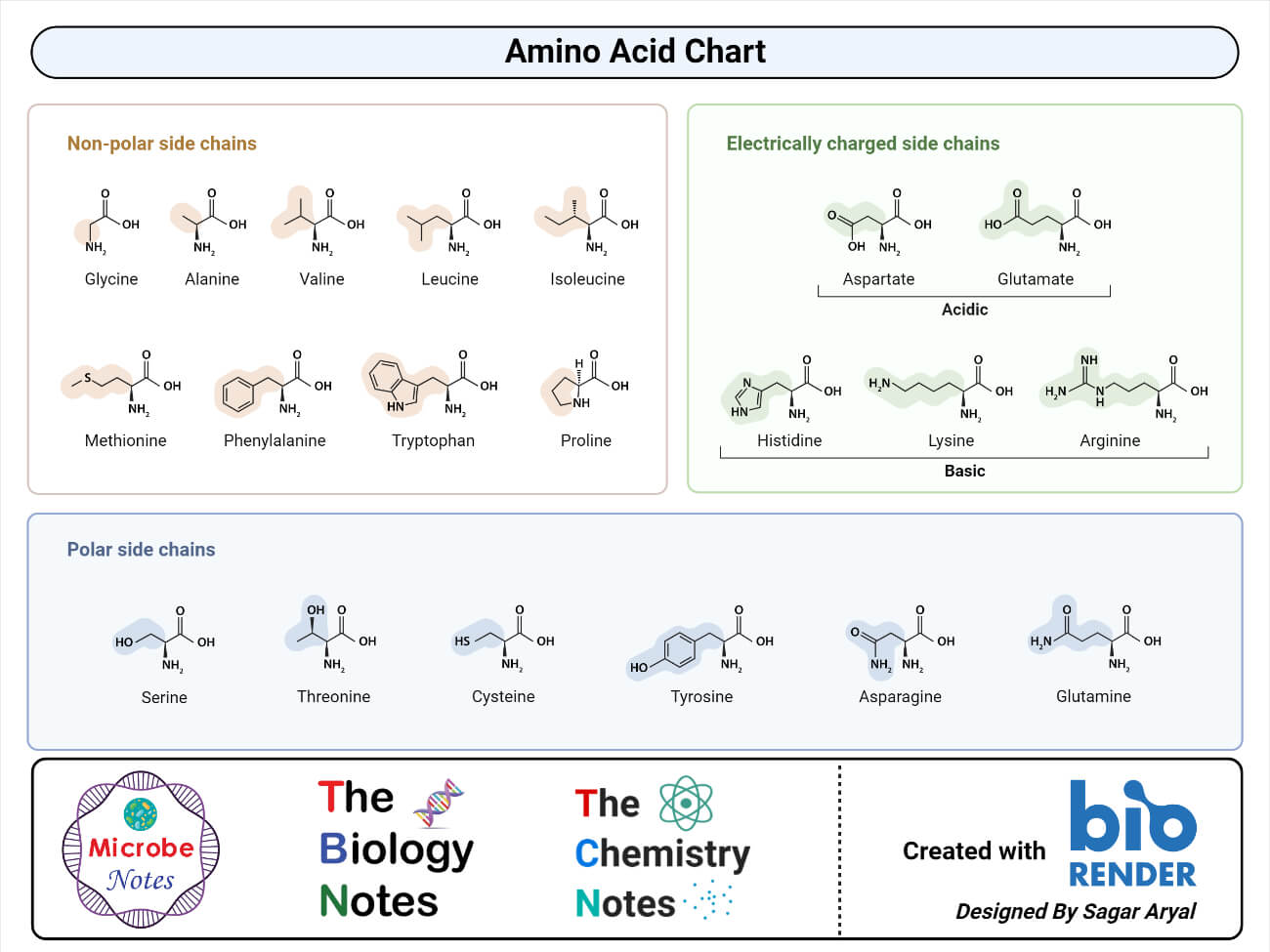
Proteins are macromolecules made up of monomers called amino acids. Amino acids are the building block of all proteins.
- An amino acid is a simple organic compound consisting of a basic group (-NH2), an acidic group (-COOH), and an organic R group that is unique to each amino acid.
- The term amino acid is short for alpha-amino carboxylic acid. Each molecule has a central carbon atom, called the α-carbon to which both the groups are attached.
- The remaining two bonds for the central carbon are satisfied by the hydrogen atom and an organic R group.
- The organic R group can be as simple as a hydrogen atom (H) or a methyl group (— CH3) or a more sophisticated group.
- Thus, the α -carbon in all the amino acids is asymmetric except in glycine where the α -carbon is symmetric with a hydrogen atom as an R group.
- Because of this asymmetry, the amino acids (except glycine) exist in two optically active forms: those having — NH2 group to the right are designated as D-forms, and those having — NH2 group to the left as L-forms.
- The property to exist in two optically different forms is termed as chirality.
- Amino acids are amphoteric compounds with both acidic and alkaline groups. These also always exist as ions except at the isoelectric point.
The general formula for an amino acid is:
COOH-CH-NH2
|
R
Interesting Science Videos
What are Proteins?
Proteins are highly complex macromolecules consisting of one or more long chains of amino acids linked together by peptide bonds.
- A protein is a macronutrient that is present in all living beings and is directly involved in various metabolic pathways.
- Proteins are species-specific and are unique to each organism. Similarly, these are also organ-specific in that the proteins of the brain are different from those in the muscles.
- Proteins are made up of 20 different amino acids, and the property of a protein molecule is a function of the amino acids present.
- Plants are capable of synthesizing all amino acids necessary to make proteins, whereas animals cannot.
- Amino acids in proteins are linked together by peptide bonds that are formed between the NH2 group of one amino acid to the COOH group of another amino acid.
- Proteins are also termed polypeptides, as they are long chains of amino acids connected by peptide bonds.
Synthesis of peptides
- A peptide is a short-chain made up of amino acid which, together with other peptides, forms a protein.
- Peptide chains are formed when two or more amino acids are connected to each other by peptide bonds.
- A peptide chain can have as few as two amino acids. Longer peptides called polypeptides have fifty to hundred amino acids.
- Peptides are categorized into different groups based on the number of amino acids present in the peptide; peptides with two amino acids are termed dipeptides whereas, peptides with more than ten amino acids are termed polypeptides.
- The peptide bond formed in proteins is a special type of amide bond that exists between two molecules where an α-carboxyl group of one molecule combines with the α-amino group of another molecule.
- The resulting chain of amino acids is thus called a peptide.
Peptide bond Formation
A peptide bond is formed between two amino acid molecules where the α-carboxyl group of one amino acid molecule reacts with the α-amino group of an adjacent molecule, resulting in a special type of amide bond.
Condensation
- The reaction involved in the formation of the peptide bond is an example of a condensation reaction resulting in dehydration (removal of water).
- The formation of a peptide bond begins as the carboxyl group of one amino acid moves towards the amino group of another amino acid.
- During the process, a hydroxyl group (OH) is lost from the carboxyl group (COOH) of the first amino acid whereas, one hydrogen is lost from the amino group (NH2) of the other amino acid.
- Thus, a water molecule (H2O) is released along with the formation of an amide bond (C-N) between the two amino acids.
- The formation of a single peptide bond between two amino acids results in a dipeptide molecule.
- Peptide bond formation is a dehydration synthesis reaction as this process involves the removal of a water molecule along with the synthesis of proteins.
Hydrolysis
- Peptide hydrolysis is an essential process in some synthetic reactions where amino acids in one peptide are cleaved and transferred to another peptide, resulting in the synthesis of a separate peptide chain.
- Peptide bond hydrolysis is also one of the mechanisms of peptide bond degradation. It involves the cleavage of polypeptides into smaller peptides or the degradation of smaller peptides into individual amino acid molecules.
- Hydrolysis of the peptide bond is catalyzed by the presence of acid and involves the addition of a water molecule.
- During hydrolysis, a water molecule is inserted between the CO-NH bond in the peptide sequence. As a result, two amino acids are separated with terminal NH2 group in one and COOH group in another.
What are polypeptides?
- Polypeptides are long chains of amino acids where more than ten amino acids are linked together by peptide bonds.
- One or more polypeptides linked together form a protein.
- One end of a polypeptide has a free amino group called the amino-terminal or N-terminal, whereas the other end has a free carboxyl group called the carboxyl-terminal or C-terminal.
- The sequence of amino acids in a polypeptide is determined by the codons present in the messenger RNA from which the polypeptide is translated.
- The sequence of codons in mRNA, in turn, is dependent on the nucleotide sequences in the DNA molecule.
Properties of Proteins
Solubility in Water
- The relationship of proteins with water is complex.
- The secondary structure of proteins depends largely on the interaction of peptide bonds with water through hydrogen bonds.
- Hydrogen bonds are also formed between protein (alpha and beta structures) and water. The protein-rich static ball is more soluble than the helical structures.
- At the tertiary structure, water causes the orientation of the chains and hydrophilic radicals to the outside of the molecule, while the hydrophobic chains and radicals tend to react with each other within the molecule (hydrophobic effect).
Denaturation and Renaturation
- Proteins can be denatured by agents such as heat and urea that cause the unfolding of polypeptide chains without causing hydrolysis of peptide bonds.
- The denaturing agents destroy secondary and tertiary structures, without affecting the primary structure.
- If a denatured protein returns to its native state after the denaturing agent is removed, the process is called renaturation.
Some of the denaturing agents include
Physical agents: Heat, radiation, pH
Chemical agents: Urea solution which forms new hydrogen bonds in the protein, organic solvents, detergents.
Coagulation
When proteins are denatured by heat, they form insoluble aggregates known as coagulum. All the proteins are not heat coagulable, only a few like the albumins, globulins are heat coagulable.
Isoelectric point
- The isoelectric point (pI) is the pH at which the number of positive charges equals the number of negative charges, and the overall charge on the amino acid is zero.
- At this point, when subjected to an electric field the proteins do not move either towards anode or cathode, hence this property is used to isolate proteins.
Molecular Weights of Proteins
- The average molecular weight of an amino acid is taken to be 110.
- The total number of amino acids in a protein multiplied by 110 gives the approximate molecular weight of that protein.
- Different proteins have different amino acid compositions and hence their molecular weights differ.
- The molecular weights of proteins range from 5000 to 109 Daltons.
Posttranslational modifications
- It occurs after the protein has been synthesized on the ribosome.
- Phosphorylation, glycosylation, ADP ribosylation, methylation, hydroxylation, and acetylation affect the charge and the interactions between amino acid residues, altering the three-dimensional configuration and, thus, the function of the protein.
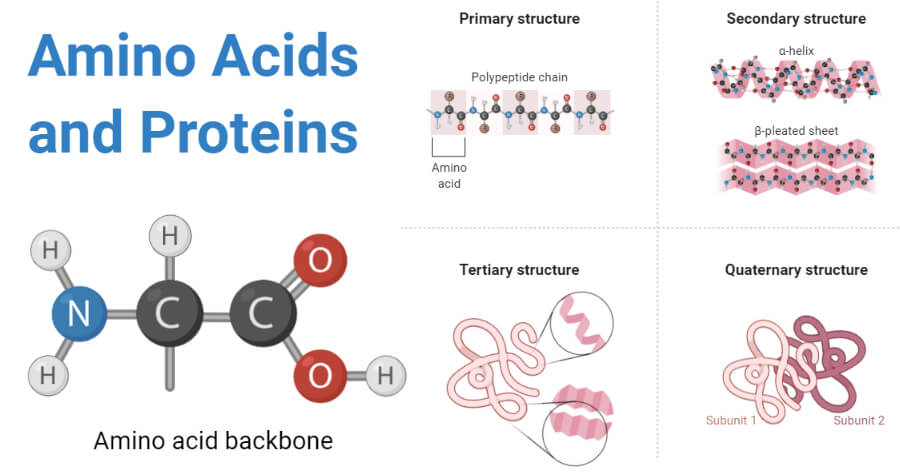
Protein Structure
- Because protein is a complex macromolecule, its structure has been described using four basic structural levels of the organization.
- These structural levels are often referred to as primary, secondary, tertiary, and quarternary.
- Three of these structural levels (primary, secondary, and tertiary) can exist in molecules composed of a single polypeptide chain. In contrast, the fourth (quarternary) involves interactions of polypeptides within a multi chained protein molecule.
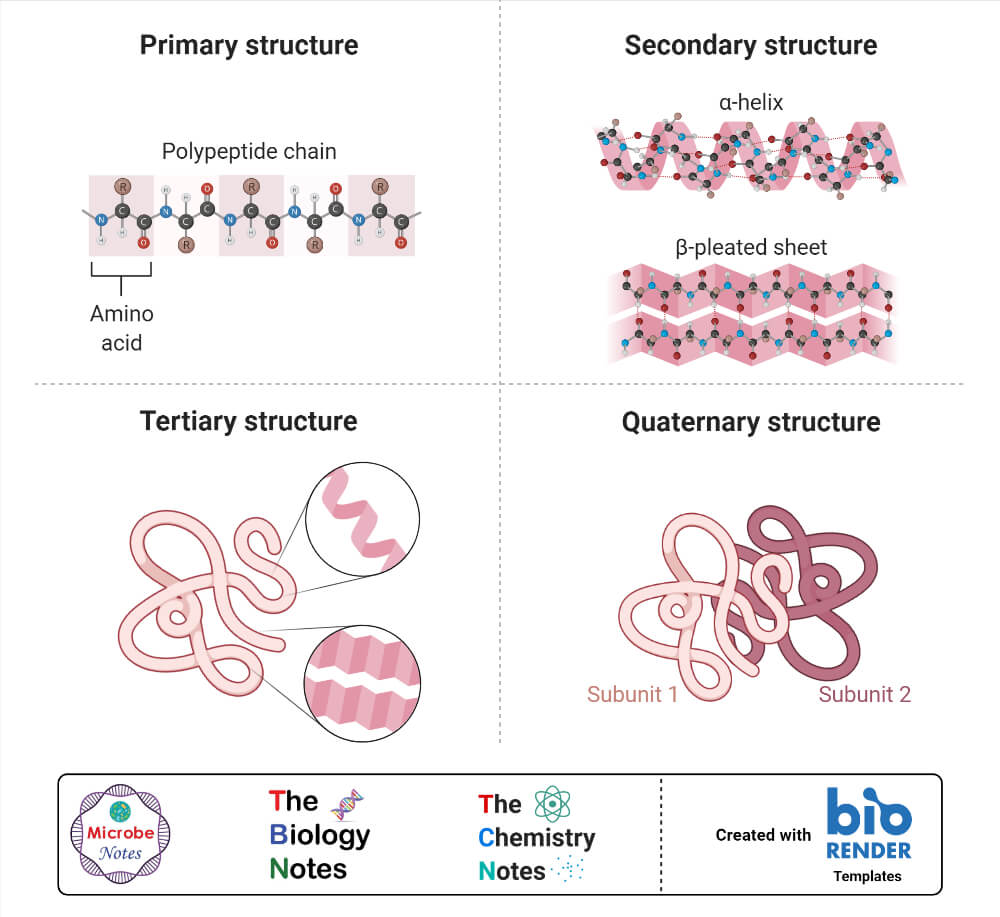
1. Primary structure
- The basic primary structure of a protein is relatively simple and consists of one or more linear chains of a number of amino acid units.
- The primary structure of a protein indicates the number and sequence of amino acids which form the constituent units of the polypeptide chain.
- The primary bond between the amino acids in proteins is the peptide bond which links the α-carboxyl group of one amino acid residue to the α-amino group of the adjacent amino acid. The proteins may consist either of one or more polypeptide chains.
- A partial double bond is created between carbon and nitrogen of the amide bond, which aids in the stabilization the peptide bond.
- A resonance effect is created as the nitrogen involved in the bond donates its lone pair to the carbonyl group.
- The resonance mechanism is highly stabilizing as the electrons can be delocalized over multiple atoms generating a resonance structure which is stable than the native structure.
- The resonance structure thus created stabilizes the bond but also limits the rotation of the amide bond due to the partial double bond.
- Peptide bonds create a planar configuration that undergoes minimal movement around the C-N bond but the other single bonds on either side of C-N bond exhibit a high degree of rotational motion.
2. Secondary structure
- The linear, unfolded primary structure of the polypeptide chain often assumes a helical shape to produce the secondary structure.
- The secondary structure of proteins refers to the steric or spatial relationship of amino acids that are near to each other in the amino acid sequence.
- The folding of the chain is mainly due to the presence of hydrogen bonds which can either be intramolecular or intermolecular.
- The folding and hydrogen bonding between neighboring amino acids results in the formation of a rigid and tubular structure called a helix.
- Secondary structures in proteins are of two types depending on the nature of the hydrogen bonding; α-helix and β-pleated sheet.
a. Alpha-helix structure
- The alpha-helix structure is formed when the CO group of each amino acid is hydrogen-bonded to the NH group of the amino acid that is present four residues ahead in the linear sequence.
- The α-helical structure depends on the intramolecular hydrogen bonding between the NH and CO groups of peptide bonds.
b. Beta-pleated structure
- The β-pleated sheet structure is formed by the parallel alignment of a number of polypeptide chains, with hydrogen bonds between the >C = O and N-H groups of adjacent chains.
- The R groups of the constituent amino acids in one polypeptide chain alternately project above and below the plane of the sheet.
- The formation of β-pleated sheets depends on intermolecular hydrogen bonding, although intramolecular hydrogen bonds are also present.
3. Tertiary structure
- The tertiary structure of the protein molecule is a three-dimensional structure of protein formed by the folding of secondary structure in certain specific patterns.
- The tertiary structure is generally stabilized by outside polar hydrophilic hydrogen and ionic bond interactions, and internal hydrophobic interactions between nonpolar amino acid side chains.
- Based upon their tertiary structure, proteins are often divided into globular or fibrous types.
a. Fibrous proteins
- Fibrous proteins have elongated rope-like structures that are strong and hydrophobic and usually consist mainly of a single type of secondary structure.
- The structures that provide support, shape, and external protection to vertebrates are made of fibrous proteins like α-keratin.
b. Globular proteins
- Globular proteins often contain several types of secondary structure and are more spherical and hydrophilic.
- Thus, most enzymes and regulatory proteins like immunoglobulins are globular proteins.
4. Quaternary structure
- The three-dimensional arrangement of protein subunits in proteins containing two or more identical or different polypeptide chains, or subunits is the quaternary structure.
- The subunits are held together by noncovalent forces between complementary surface hydrophobic and hydrophilic regions on the polypeptide subunits.
- These forces allow the polypeptide chains to undergo rapid conformational changes that affect the biological activity of the proteins.
Protein Bonding
- Besides the primary peptide bonds, several other secondary bonds are responsible for the formation of the stable net structure of proteins.
- Some of the common secondary bonds present within proteins are:
1. Hydrogen Bonds
- A hydrogen bond is formed in proteins because of the tendency of a hydrogen atom covalently bonded to an electronegative atom to share electrons with the adjacent atoms like O or N.
- In a peptide bond, hydrogen bond can be observed as below:
-C=O∙∙∙∙∙∙∙∙∙∙∙∙HN-
The dotted line between oxygen and hydrogen atoms in peptide linkage represents a hydrogen bond.
- Hydrogen bond in protein is important as it stabilizes the secondary structure of proteins.
2. Ionic Bonds
- Ionic bonds in proteins are observed between the acidic and basic groups of the constituent amino acids.
- Electrostatic interactions also exist between differently charged groups present on the side chains of amino acids.
- Ionized groups are involved in stabilizing interactions between protein and other molecules rather than stabilizing the protein structure.
- These ionic bonds, although weaker than the hydrogen bonds, are responsible for maintaining the three-dimensional structure or the tertiary structure of the globular proteins.
3. Disulfide Bonds
- The disulfide bond is the second type of covalent bond found between amino acid residues in proteins and polypeptides.
- This bond is formed by the oxidation of the thiol or sulfhydryl (-SH) groups of two cysteine residues to yield cysteine.
- Even if the disulfide bridges are very strong when compared to the strength of noncovalent bonds, they are of short-range and can only stabilize the tertiary structure once the bond is completely formed.
Hydrophobic and Hydrophilic interactions
- Hydrophobic interactions occur between the side chains or essentially hydrophobic R groups.
- Hydrophobic groups unite among themselves, causing the elimination of water to form linkages between various segments of a chain or between different chains.
- Hydrophobic interactions might also lead to other bonds like hydrogen bonds or ionic bonds between other groups.
- The hydrophobic bonds also aid other protein interactions, for example, the formation of enzyme-substrate complexes and antibody-antigen interactions.
- Hydrophilic interactions result in hydrogen bonding between electronegative atoms and hydrogen atoms.
Fibrous Proteins
1. Collagen
- Collagen is the most abundant protein of mammals that makes up about 25-33% of all the body proteins.
- It is the principal structural element of the human body that is found in connective tissues such as tendons, cartilage, the organic matrix of bones, and the cornea of the eye.
- Structurally, the collagen helix is a unique secondary structure quite distinct from the α helix. It is left-handed and has three amino acid residues per turn.
- Collagen is also a coiled-coil, but with distinct tertiary and quaternary structures where three separate polypeptides, called α chains are supertwisted about each other.
- Typically they contain about 35% glycine, 11% alanine, and 21% proline, and 4-hydroxyproline.
2. Keratin
- Keratin or α-keratin is a fibrous protein that constitutes almost the entire dry weight of hair, wool, nails, claws, quills, horns, hooves, and much of the outer layer of skin in mammals.
- The α-keratins are part of a broader family of proteins called intermediate filament (IF) proteins.
- The α-keratin helix is a right-handed α-helix, the secondary structure found in many other proteins.
- Two strands of α-keratin, oriented in parallel, are wrapped about each other to form a super twisted coil that amplifies the strength of the overall structure.
- An individual polypeptide in the α -keratin coiled-coil has a relatively simple tertiary structure, dominated by an α -helical secondary structure with its helical axis twisted in a left-handed superhelix.
- The intertwining of the two α -helical polypeptides in keratin acts as an example of a quaternary structure.
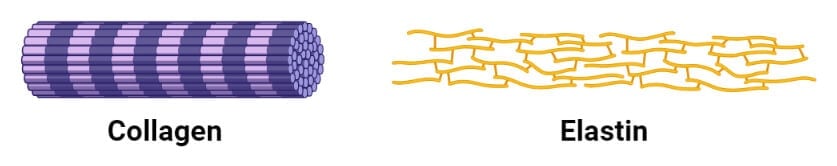
3. Elastin
- Elastin is a major protein found in various organs that require elasticity like lungs, bladder, and elastic cartilage.
- The polypeptide chain consists of tropoelastin protein, containing glycine and valine and modified alanine and proline residues.
- It is classified as a fibrous protein due to its structural function and insolubility in water.
- Elastin lacks a regular secondary structure and has cross-linkages of various protein sequences.
- Elastin is also rich in glycine and proline, but it doesn’t have a glycine molecule like every third residue like in collagen.
Globular Proteins
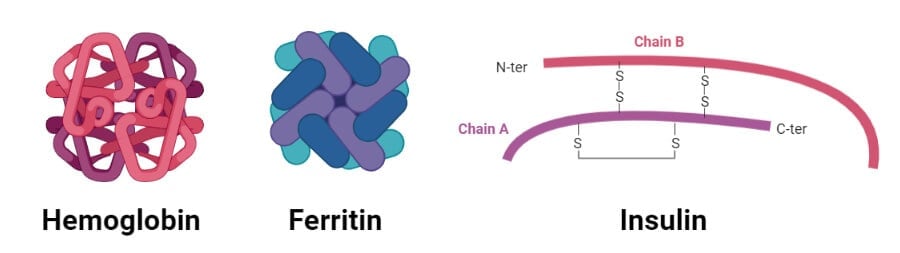
1. Hemoglobin
- Hemoglobin is the oxygen transporter in erythrocytes and constitutes about 90% of the protein of red blood cells.
- It is a tetrameric protein with four polypeptide chains held together by noncovalent interactions.
- Two of the four polypeptide chains are α chains, and the remaining two are β chains.
- The α- and β-chains of hemoglobin contain several segments of α-helix held together as a pair by ionic and hydrogen bonds.
- Similarly, the four polypeptide chains fit together almost tetrahedrally to produce the characteristic quaternary structure.
2. Insulin
- Insulin is a peptide hormone produced by the beta cells of the pancreas that regulates the metabolism of carbohydrates, fats, and proteins.
- Insulin produced in the body is stored in the form of the hexamer, but the active form is a monomer.
- The hexamer serves as a way to keep the protein stable and protected.
- A single protein monomer of insulin is composed of 51 amino acids formed by two peptide chains linked together by disulfide bonds.
- One of the chains has two α-helixes while the other chains have an α-helix and two β-sheets.
- The amino acid sequence in insulin is highly conserved and differs only slightly between different species.
3. Pepsin
- Pepsin is an enzyme that breaks down proteins into smaller peptides or amino acids.
- It is one of the three important proteases in the human body, along with trypsin and chymotrypsin.
- Pepsin is released by the cells on the stomach wall in the form of pepsinogen which upon mixing with hydrochloric acid activates into pepsin.
- The predominant secondary structure in pepsin is β-sheets along with six right-handed α-helixes.
- Pepsin is also a part of rennet that is used to curdle milk during the manufacturing of cheese.
Conjugated Proteins
1. Glycoproteins
- Glycoproteins are the proteins containing carbohydrates as a prosthetic group. They contain small amounts of carbohydrates (usually less than 4%).
- Glycoproteins act as important integral proteins in various biological membranes assisting in cell-cell interactions.
- Immunoglobulins are important glycoproteins of the immune system that act as antibodies and protect against antigens.
- Similarly, soluble glycoproteins are also found in egg albumin and blood plasma.
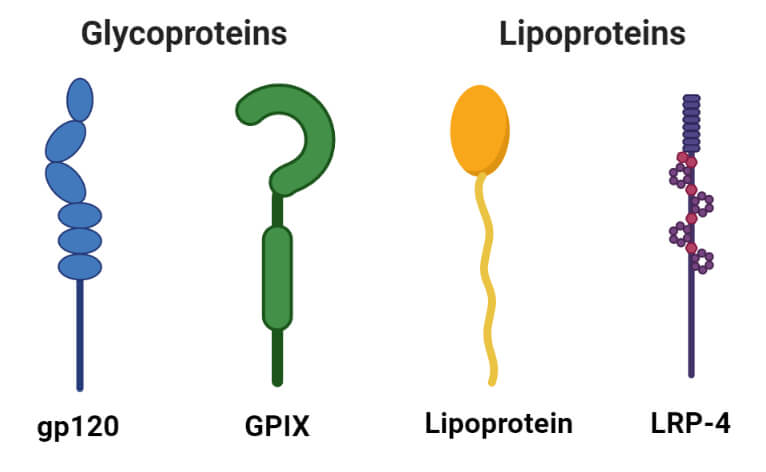
2. Lipoproteins
- Lipoproteins are proteins forming complexes with lipids like cephalin, lecithin, and cholesterol.
- These are soluble in water but insoluble in organic solvents.
- The lipoproteins act as the temporary intermediates in the process of transfer of lipids from the site of absorption to the site of utilization.
- Lipoproteins are classified into four groups based on the migration of their fraction in a density gradient separation technique.
Protein Denaturation
- Protein denaturation is the process of destruction of the quaternary, tertiary, and secondary structure of proteins by the application of external force or strong chemicals like acid and base.
- Denaturation of protein might destroy cell activity as most of the metabolic pathways require proteins in one form or the other.
- After denaturation, proteins exhibit a range of properties like conformation change and loss of solubility.
- Hydrogen bonds responsible for the tertiary structure of proteins are weak and break easily with heat and radiation.
- Denaturation, however, doesn’t affect the primary structure of proteins.
- In some cases, denaturation is reversible, and the proteins can regain their native form once the stressor is removed. However, some cases of denaturation are irreversible.
Classification of Proteins
Based on the chemical nature, structure, shape, and solubility, proteins are classified as:
- Simple proteins: They are composed of only amino acid residue. On hydrolysis, these proteins yield only constituent amino acids. It is further divided into:
- Fibrous protein: Keratin, Elastin, Collagen
- Globular protein: Albumin, Globulin, Glutelin, Histones
- Conjugated proteins: They are combined with non-protein moiety. Eg. Nucleoprotein, Phosphoprotein, Lipoprotein, Metalloprotein, etc.
- Derived proteins: They are derivatives or degraded products of simple and conjugated proteins. They may be :
- Primary derived protein: Proteans, Metaproteins, Coagulated proteins
- Secondary derived proteins: Proteosesn or albunoses, peptones, peptides.
Functions of Proteins
Proteins are essential biomolecules that are critical to life and to perform various activities. Some of the important biological roles of proteins are:
- Many proteins act as catalysts that enhance the rate of chemical reactions in various metabolic pathways.
- The fibrous proteins are a component of various tissues holding the skeletal elements together like collagen, which is a structural unit of connective tissues.
- The nucleoproteins serve as carriers of genetic characters and hence govern the inheritance of traits.
- Proteins also perform transport functions that regulate the transport of many compounds in and out of the cells and accumulate inside at much higher concentrations than expected from diffusion alone.
- Various protein hormones regulate the growth of plants and animals, besides controlling many other physiological functions.
- Blood plasma has multiple soluble proteins that can be used for the treatment of shock produced by severe injuries and operations.
- Interferons are regulatory glycoproteins produced by many eukaryotic cells in response to virus infection, endotoxins, antigenic stimuli, and many parasitic organisms.
- Peptides from humans called defensins are antibiotic in nature.
References and Sources
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- https://chemistry.tutorvista.com/biochemistry/proteins.html
- http://www.biologydiscussion.com/proteins/proteins-definition-importance-and-classification-biochemistry/41903
- https://www.particlesciences.com/news/technical-briefs/2009/protein-structure.html
- http://www.biologydiscussion.com/proteins/proteins-functions-structure-properties-and-classification/16912
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- 2% – https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/tertiary-structure
