Respiration is a metabolic biochemical reaction wherein the complex food material is converted into the form of cellular energy molecules (ATP) inside living cells. It is a series of oxidation-reduction reactions where an electron is transformed from an electron donor to an acceptor, and ultimately energy is released and conserved in form of the adenosine triphosphate (ATP) molecule. In general concept, respiration is considered inhaling oxygen and exhaling carbon dioxide gas. But, this is termed breathing instead of respiration.
Based on oxygen requirement, respiration can be classified into two types; aerobic and anaerobic respiration. More precisely, this classification is based on the use of oxygen or any other molecules as the electron acceptor in the respiratory process.
Anaerobic respiration is the cellular respiration process that takes place in the absence of oxygen. It is the process where an electron is transferred to molecules other than an oxygen molecule. It is a quicker method, but there is an incomplete breakdown of glucose, releasing fewer ATP molecules. It mainly occurs in microorganisms.
Interesting Science Videos
What is Aerobic Respiration?
Aerobic respiration is the cellular respiration process that occurs in the presence of oxygen.
It is the respiratory process where the electron is transferred to dioxygen molecules (O2), generating water molecules and energy molecule ATP. Here glucose molecule is completely oxidized into energy (ATP), carbon dioxide, and water. The general process of aerobic respiration can be expressed as:
C6H12O6 (Glucose) + O2 (Oxygen) → 6CO2 (Carbondioxide) + 6H2O (water) + ATP (Energy)
One molecule of glucose is oxidized to 6 molecules of carbon dioxides, 6 molecules of water, and 32 molecules of ATP.
It occurs in most living organisms, including every higher plant and animal, and most microorganisms in the aerobic and facultative mode of respiration. It occurs in the mitochondria of eukaryotes and the cytoplasm of prokaryotes’ cells. It is slower compared to the anaerobic type but has a higher yield of ATP molecules. A total of 32 ATP molecules are generated from a single glucose molecule. In this process, glucose, amino acids, and fatty acids are used as substrates and metabolized in the presence of oxygen.
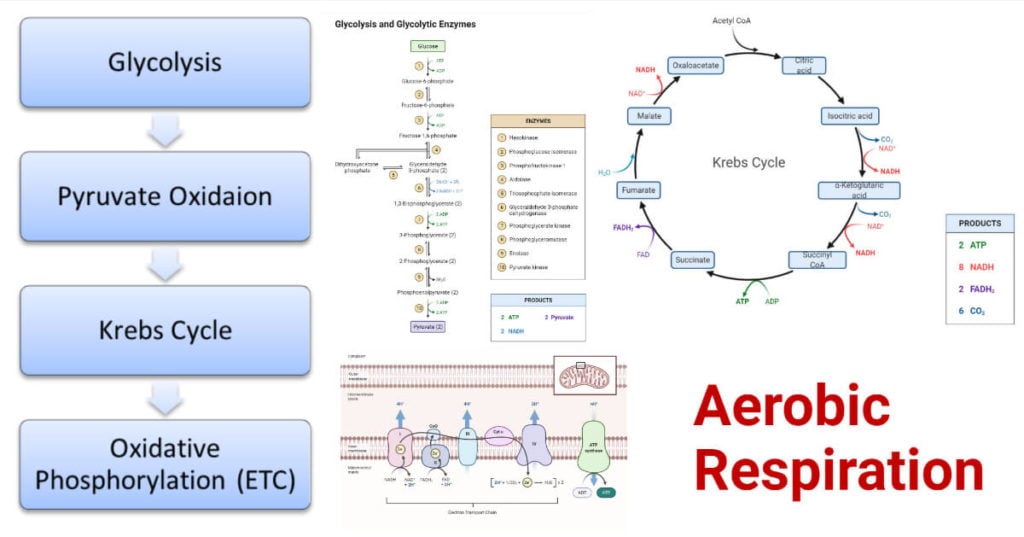
Steps of Aerobic Respiration
It is a complex multistep enzymatic reaction process that occurs in four steps; glycolysis, pyruvate oxidation, Krebs cycle, and oxidative phosphorylation. Each step is already a complex multistep enzymatic chemical reaction process.
Glycolysis
Etymology: Derived from the Greek words “glycose” meaning “sugar”, and “lysis” meaning “dissolution”.
Glycolysis is the sequence of catabolic reactions converting glucose (or glycogen) molecule to pyruvic acid using several enzymes. In the process, a glucose molecule is catalyzed to yield two molecules of pyruvic acids, two molecules of NADH, and two molecules of ATP. It is also called “Embden-Meyerhof-Parnas Pathway” or “EMP Pathway”.
The overall reaction can be expressed as:
Glucose + 2NAD+ +2ADP + 2Pi → 2Pyruvate + 2NADH + 2ATP + 2H2O + 2H+
It occurs in the cytoplasm of every cell of a body. It occurs in both aerobic and anaerobic conditions. In aerobic conditions, pyruvic acid is produced, whereas lactic acid is produced in anaerobic conditions.
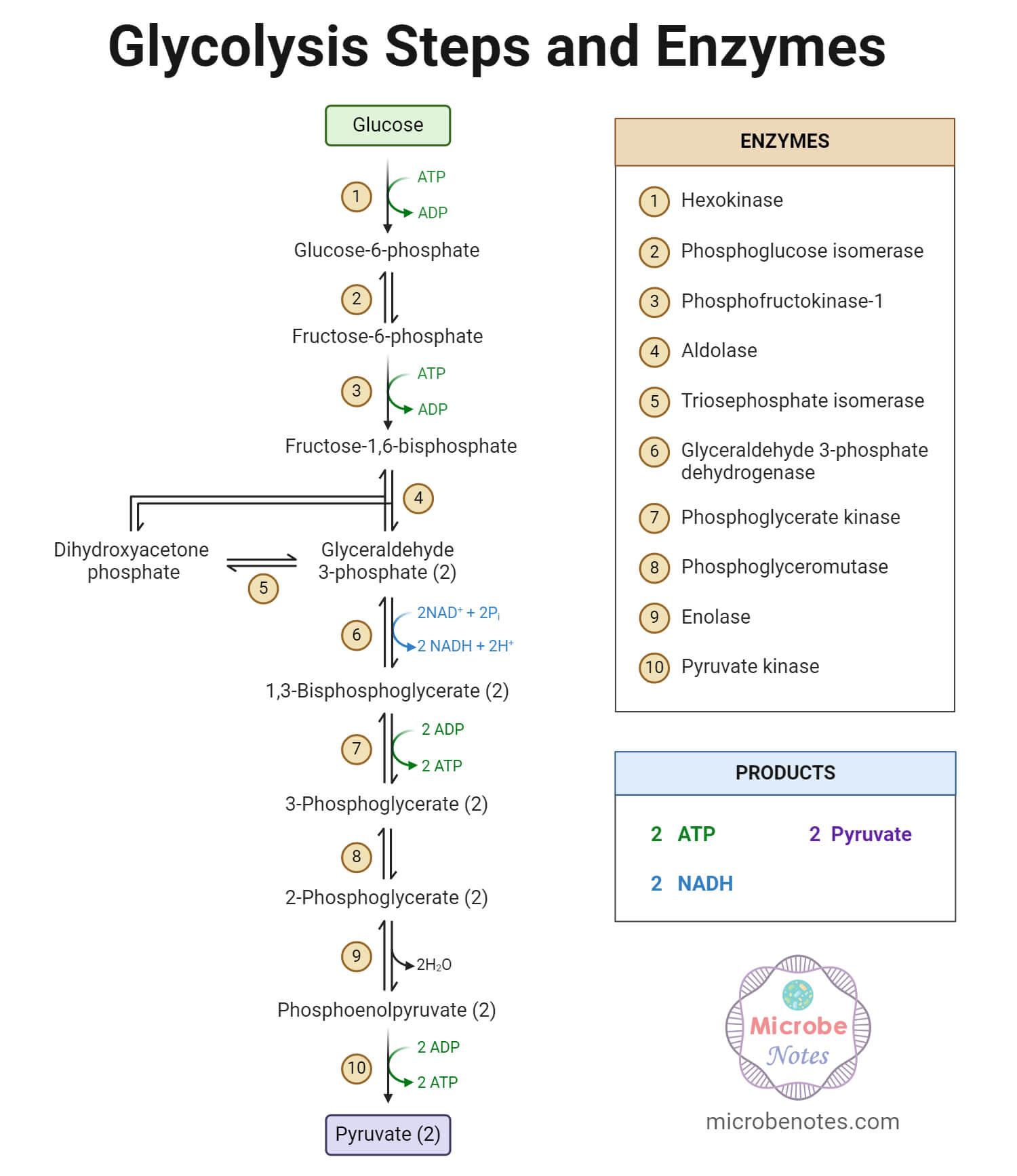
It is the first step of the aerobic respiration process. It is a multistep reaction process. The sequence of reaction can be divided into three distinct phases;
A. Energy Investment Phase (Preparatory Phase)
It is the first phase where glucose is converted to Fructose-1, 6-bisphosphate using two molecules of ATP (hence called the energy investment phase) in a three-step reaction, viz.:
- Phosphorylation of glucose: Here glucose is phosphorylated to glucose – 6 – phosphate by the enzyme “hexokinase” using an ATP molecule.
- Isomerization of glucose – 6 – phosphate (G-6-P): Here, the enzyme “glucose – 6 – phosphate isomerase” catalyzes the reversible isomerization of G-6-P to fructose – 6 – phosphate in presence of Mg+2 ion.
- Phosphorylation of fructose – 6 – phosphate (F-6-P): Here, F-6-Pis phosphorylated by the “phosphofructokinase” enzyme to yield fructose- 1,6 – bisphosphate. It is called the committed step of glycolysis.
B. Splitting Phase
It is a two-step reaction where the 6-carbon fructose – 1,6 – bisphosphate molecule is split into two 3 – carbon compounds glyceraldehyde – 3 – phosphate.
- Cleavage of fructose – 1,6 – bisphosphate: Fructose – 1,6 – bisphosphate is converted to two triose phosphate; glyceraldehyde – 3 – phosphate (G – 3 – P) and dihydroxyacetone phosphate, by an enzyme called “aldolase (fructose bisphosphate aldolase)”.
- Triosephosphate isomerization: The enzyme “triosephosphate isomerase” reversibly converts dihydroxyacetone phosphate to G – 3 – P.
C. Energy Generation Phase
It is the final phase where the glyceraldehyde – 3 – phosphate molecules are converted to pyruvate. It comprises the following reaction sequence:
- Oxidation of glyceraldehyde – 3 – phosphate (G-3-P): G – 3 – P is oxidized to 1,3 – bisphosphoglycerate by the enzyme “glyceraldehyde – 3 – phosphate dehydrogenase” producing a molecule of NADH.
- Phosphoryl Transfer from 1,3 – bisphosphate to ADP: Enzyme “phosphoglycerate kinase” reversibly catalyze the conversion of 1,3 – bisphosphate to 3 – phosphoglycerate. A molecule of ATP is synthesized in the process.
- Conversion of 3 – phosphoglycerate to 2 – phosphoglycerate: The enzyme “phosphoglycerate mutase” catalyzes the conversion of 3 – phosphoglycerate to 2 – phosphoglycerate.
- Dehydration of 2 – phosphoglycerate: In a reversible reaction, 2 – phosphoglycerate is dehydrated to phosphoenolpyruvate by the enzyme “enolase”.
- Formation of Pyruvate: The final transfer of the phosphoryl group from phosphoenolpyruvateto ADP yields a pyruvate molecule. It is catalyzed by the enzyme “pyruvate kinase”.
Pyruvate Oxidation
It is the second step where pyruvate is converted to Acetyl-CoA which can then enter the Krebs cycle. This step results in the oxidative decarboxylation of pyruvate produced by glycolysis.
First, the pyruvate is transferred to the mitochondrial matrix by the “pyruvate translocase” enzyme. The enzyme “pyruvate dehydrogenase complex” irreversibly catalyzes the conversion of pyruvate to Acetyl – CoA. There is a loss of an atom of carbon from pyruvate, forming a molecule of CO2. Additionally, NAD+ is reduced to NADH in the process.
The overall reaction can be summarized as:
Pyruvate + NAD+ + CoA → Acetyl CoA + CO2 + NADH + H+
Krebs Cycle
It is a sequence of reactions where Acetyl – CoA is oxidized to CO2 and H2O inside the mitochondrial matrix generating energy in the form of ATP and reduced coenzymes NADH and FADH2. 20 ATP molecules are produced in this stage. This is the central aerobic pathway that connects all the individual metabolic pathways and utilizes about 2/3rd of the total oxygen consumed by the body.
It was discovered by German biologist and biochemist Sir Hans Adolf Krebs, hence got the name “Krebs cycle”.
It occurs in the mitochondrial matrix because all the required enzymes are located inside the mitochondria. It has catabolic and anabolic reactions, hence regarded as a cyclic amphibolic aerobic oxidative metabolic pathway. It is involved in gluconeogenesis, transamination, and delamination processes.
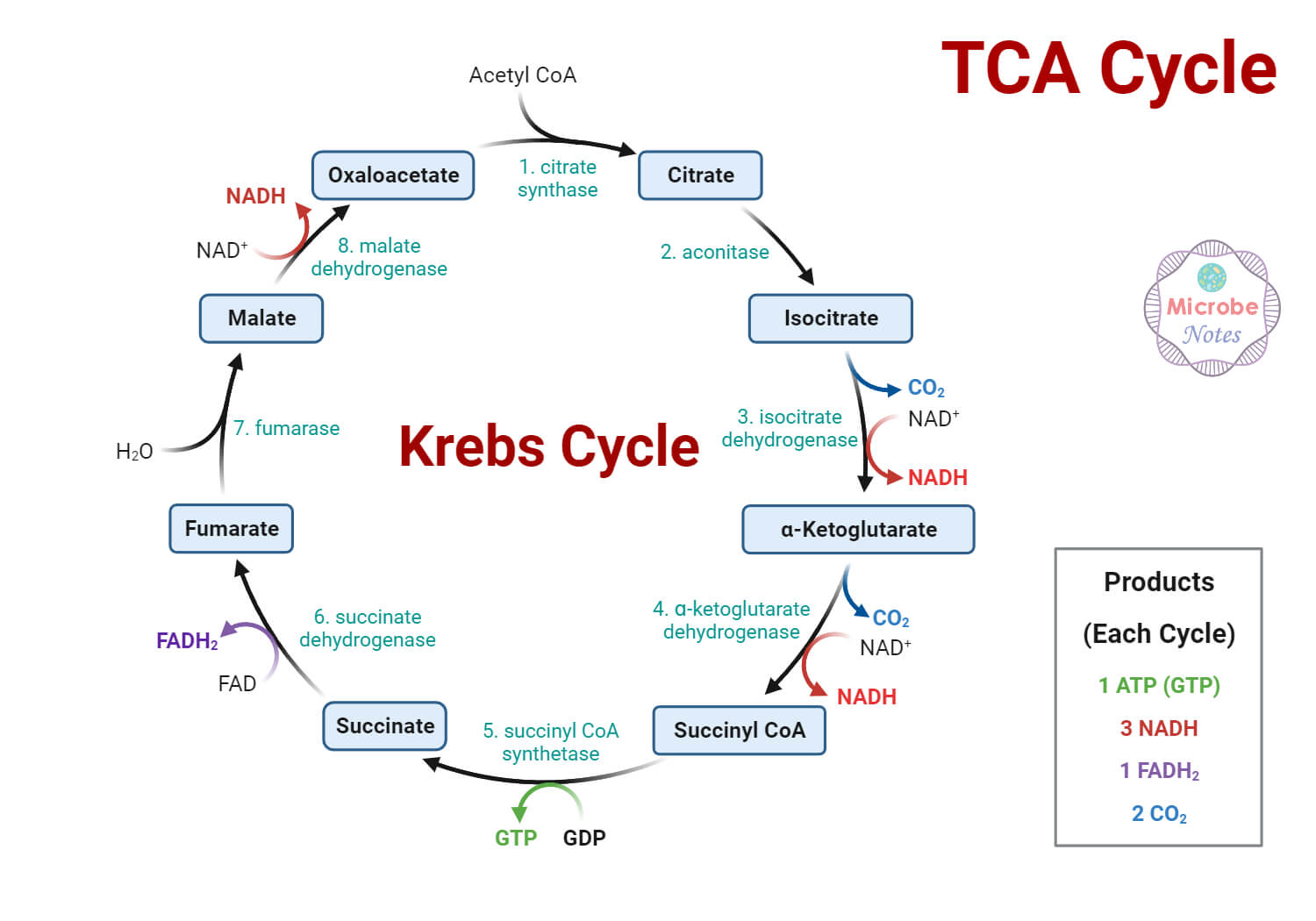
Citric acid is produced in the first step of the reaction. Hence it is also called the “Tricarboxylic Acid Cycle (TCA Cycle)” or “Citric Acid Cycle”.
It is a multistep oxidative reaction cycle including the following steps:
- Formation of Citrate: acetyl – CoA and oxaloacetate are condensed by the enzyme “citrate synthase” resulting production of citric acid.
- Isomerization of Citrate: enzyme “aconitase” isomerizes the citrate to isocitrate (isocitric acid) through an intermediate process forming cis-aconitate which further rehydrates to form isocitrate.
- Formation of – ketoglutarate: isocitrate is first dehydrogenated to oxalosuccinate and then decarboxylated to – ketoglutarate by an enzyme “isocitrate dehydrogenase”. A molecule of CO2 and NADH is formed in the process.
- Conversion of – ketoglutarate to Succinyl CoA: oxidative decarboxylation of – ketoglutarate by the enzyme – ketoglutarate dehydrogenase results in the formation of succinyl CoA and CO2. NADH is released as NAD+ serves as an electron acceptor.
- Formation of Succinate: Succinyl CoA is converted to succinate by “succinate thiokinase”. A molecule of GDP is phosphorylated to GTP which is, in turn, converted to ATP by enzyme nucleoside diphosphate kinase.
- Oxidation of Succinate: “Succinate dehydrogenase” oxidizes succinic acid (succinate) to fumarate (fumaric acid). A molecule of FADH2 is produced in the process.
- Hydration of Fumarate: “Fumarase” catalyze the hydration of fumarate to malate (malic acid).
- Oxidation of Malate: Finally, the enzyme “malate dehydrogenase” oxidize malate to oxaloacetic acid (oxaloacetate). The third and final synthesis of NADH occurs in this stage.
Oxidative Phosphorylation
It is the series of biochemical reactions catalyzed by several enzymes, in which electrons are transferred from electron carriers like NADH and FADH to an oxygen molecule (O2), releasing ATP molecules in the process.
Generally, oxidative phosphorylation is understood as an electron transport chain. Broadly, oxidative phosphorylation is coupled reaction series including electron transport chain (ETC) and chemiosmosis.
The ETC transfers the electrons and generates a proton gradient, and the ATP is synthesized by using ATP synthase, ADP, and the energy generated by the proton gradient in a process called chemiosmosis.
It occurs inside the mitochondria in its inner membrane. It is the final step in aerobic cellular respiration.
A. Electron Transport Chain
The ETC is a series of complexes that transfer the electrons from the electron donors (NADH and FADH) to the electron acceptors (O2) via oxidation-reduction reactions, and couples this electron transfer with the transfer of proton (H+) across the mitochondrial membrane.
ETC involves five distinct enzyme complexes denoted I, II, III, IV, and V. The complexes I – IV are electron carriers while complex V is responsible for ATP synthesis. Besides, there are other mobile electron carriers like NADH, coenzyme – Q, Cytochrome – C, and oxygen.
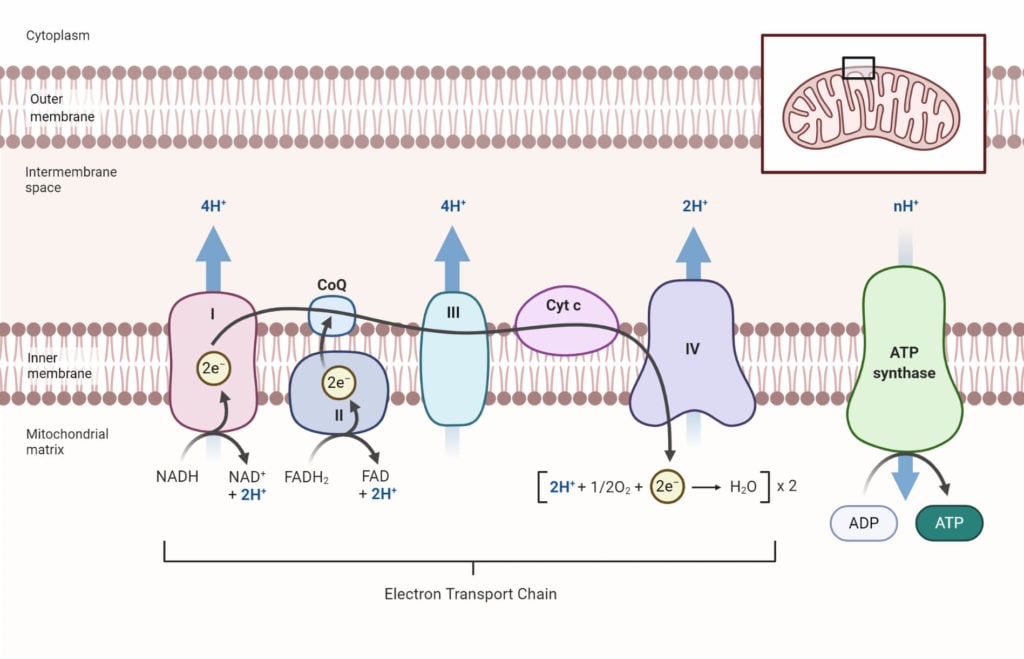
The overall reactions in the ETC can be listed as:
1. Delivery of electrons; substrates like NADH and FADH transfer their electrons to the prosthetic group FMN coenzyme. The coenzyme FMN accepts 2 electrons and a proton to form FMNH2.
NADH + H+ + FMN → NAD+ + FMNH2
2. Transport of electrons; one iron-sulfur (FeS) protein participates in electron transfer from FMNH2 to coenzyme Q. There are 6 FeS identified that exist in either oxidized Fe3+ or reduced Fe2+ form. All these FeS proteins are associated with cytochromes (b, c1, c, a, a3) and take part in transporting electrons.
3. Formation of the water molecule; in the final stage, the transported electrons are accepted by the oxygen molecule. This splits the O2 into two O– radicles and combines with the free proton (H+) to form a water molecule. This reaction is exergonic and produces energy which is used to produce ATP.
B. Chemiosmosis
It is the process where the energy generated from the proton gradient is used to produce ATP. The transfer of electrons in the ETC is coupled with the translocation of the proton (H+) which develops a proton gradient.
The movement of H+ ions down their electrochemical gradient activates the ATP synthase enzyme which will catalyze the phosphorylation of ADP forming ATP molecule.
ATP Generation in Aerobic Respiration
During the aerobic respiration process, ATPs are generated in oxidative phosphorylation at two levels; first at the substrate level, and the second at the ETC level.
The first is called substrate-linked oxidative phosphorylation, occurring in the glycolytic pathway and TCA cycle. In the glycolytic pathway, oxidation of G-3-P by G-3-P dehydrogenase enzyme adds a high energy phosphate group which is transferred to ADP in the next reaction generating ATP molecule.
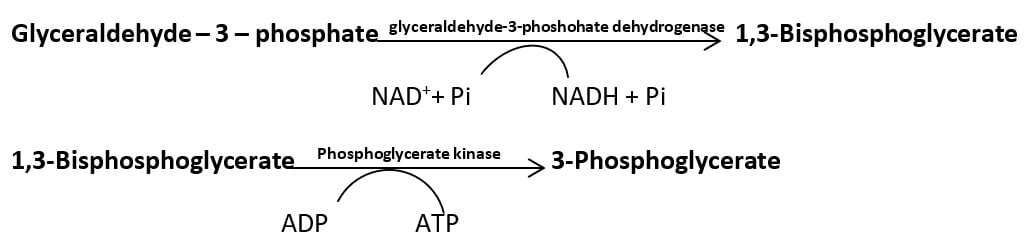
In another reaction, the energy released during dehydration of 2-phosphoglycerate converts the low energy phosphate bond into a high energy phosphate bond which is transferred to ADP in the next reaction producing an ATP molecule.

In the TCA cycle energy released during the oxidation of α-ketoglutarate is used to form a high energy bond between succinate and CoA. In the next reaction, CoA is separated, and the energy is released, which is used to phosphorylate GDP to GTP.
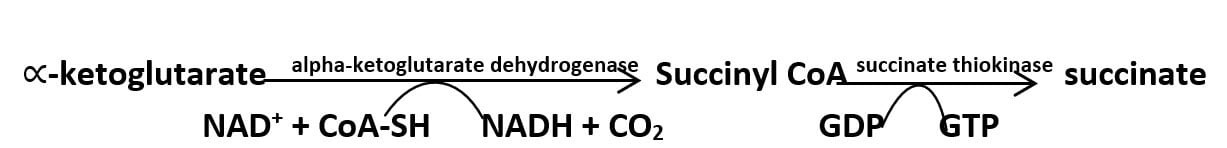
The second level is called oxidative phosphorylation at the ETC level. Reducing equivalents formed during glycolysis and TCA cycles are oxidized in the ETC coupled with the phosphorylation of ADT to generate ATP in chemiosmosis.
Total ATP yield per glucose molecule in aerobic respiration can be summarized as:
| In Glycolysis ATP consumed = 2 ATP ATP produced = 4 ATP NADH produced = 2 NADH = 2 x 2.5 = 5 ATPs |
| In Oxidative Decarboxylation of Pyruvate NADH produced = 2 NADH = 2 x 2.5 = 5 ATPs |
| In (TCA) Krebs Cycle Since 2 Acetyl CoA are formed by one glucose molecule, the number of ATP production is double of ATP produced in one TCA cycle. Total NADH produced = 2 x 3 NADH = 6 x 2.5 = 15 ATPs Total FADH2 produced = 2 x 1 FADH2 = 2 x 1.5 = 3 ATPs Total direct ATPs produced = 2 ATPs |
| In Oxidative Phosphorylation the NADH and FADH2 are converted to ATPs which are already included above. |
| TOTAL ATP YIELD = 7 (in glycolysis) + 5 (in oxidative decarboxylation) + 20 (in TCA) = 32 ATPs |
Applications of Aerobic Respiration
- First and foremost, it is used for the production of energy in the form of ATP in cells of aerobic organisms.
- CO2 released during the process is used in photosynthesis by green plants.
- Several intermediate compounds, coenzymes, and organic acids are produced.
- They are used in the production of proteins from amino acids during anabolic reactions.
- They are used in aerobic composting and biodegradation processes.
References
- Nelson, D. L., & Cox, M. M. (2017). Lehninger principles of biochemistry (7th ed.). W.H. Freeman.
- Satyanarayana, U. (2013). Biochemistry. Elsevier Health Sciences. https://books.google.com.np/books?id=Bd9XAwAAQBAJ
- Respiration- Types and Phases Of Respiration In Organisms (byjus.com)
- Respiration – Definition, Types, Flow Chart & Stages (vedantu.com)
- Respiration and gas exchange – KS3 Biology – BBC Bitesize – BBC Bitesize
- Patwa, A., & Shah, A. (2015). Anatomy and physiology of respiratory system relevant to anaesthesia. Indian journal of anaesthesia, 59(9), 533–541. https://doi.org/10.4103/0019-5049.165849
- Kelly DJ, Hughes NJ, Poole RK. Microaerobic Physiology: Aerobic Respiration, Anaerobic Respiration, and Carbon Dioxide Metabolism. In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Washington (DC): ASM Press; 2001. Chapter 10. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2411/
- Qusheng Jin, American Journal of Science June 2012, 312 (6) 573-628; DOI: https://doi.org/10.2475/06.2012.01
- (14) (PDF) Difference Between Aerobic and Anaerobic respiration (researchgate.net)
- Aerobic Respiration: Definition, Process & Significance (embibe.com)
- Aerobic Respiration – The Definitive Guide | Biology Dictionary
- Aerobic Respiration – Definition, Process and Steps | Biology (vedantu.com)
- What Is Aerobic Respiration? – Definition, Diagram and Steps (byjus.com)
- Wilson D. F. (2017). Oxidative phosphorylation: regulation and role in cellular and tissue metabolism. The Journal of physiology, 595(23), 7023–7038. https://doi.org/10.1113/JP273839
- Oxidative Phosphorylation – Definition, Oxidative Phosphorylation Steps (byjus.com)
- Importance of Aerobic Respiration and Anaerobic Respiration – QS Study
- Real-life applications – Respiration – Anaerobic Respiration, Anaerobic bacteria, Humans and anaerobic respiration (scienceclarified.com)
