The iodine test is a chemical test used to distinguish mono- or disaccharides from certain polysaccharides like amylose, dextrin, and glycogen. This test has a variation termed starch-iodine test that is performed to indicate the presence of glucose made by plants in the leaves.
Objectives of the Iodine Test
- To detect the presence of polysaccharides, primarily starch.
Principle of Iodine Test
- The iodine test is based on the fact that polyiodide ions form colored adsorption complex with helical chains of glucose residue of amylose (blue-black), dextrin (black), or glycogen (reddish-brown).
- Monosaccharides, disaccharides, and branched polysaccharides like cellulose remain colorless. Amylopectin produces an orange-yellow hue.
- The reagent used in the iodine test is Lugol’s iodine, which is an aqueous solution of elemental iodine and potassium iodide.
- Iodine on its own is insoluble in water. Addition of potassium iodine results in a reversible reaction of the iodine ion with iodine to form a triiodide ion, which further reacts with an iodine molecule to form a pentaiodide ion.
- Bench iodine solution appears brown, whereas, the iodide, triiodide, and pentaiodide ion are colorless.
- It is observed that the helix (coil or spring) structure of the glucose chain is the key to this test.
- Further, the resulting color depends on the length of the glucose chains.
- The triiodide and pentaiodide ions formed are linear and slip inside the helix structure.
- It is believed that the transfer of charge between the helix and the polyiodide ions results in changes in the spacing of the energy levels, which can absorb visible light, giving the complex its color.
- The intensity of the color decreases with the increase in temperature and the presence of water-miscible organic compounds like ethanol.
- On heating, the blue color amylose-iodine complex dissociates but is formed again on cooling because the helical structure is disrupted; thereby amylose loses its iodine binding capacity and the blue color.
- The blue color reappears on cooling due to the recovery of iodine binding capacity due to regaining of the helical structure.
Requirements
1. Reagent
- Lugol’s iodine: 5% elemental iodine is mixed with 10% potassium iodide to form the Lugol’s iodine.
- Test sample
2. Materials Required
- Test tubes
- Test tube stand
3. Equipment
- Water bath
Procedure of Iodine Test
- Take 1 ml of a given sample in a clean, dry test tube.
- Take control of 1 ml of distilled water in another tube.
- Add about 2-3 drops of Lugol’s solution to both the tubes and mix them in a vortex.
- Observe the appearance of color in the test tubes.
- Heat the test tubes in the water bath until the color disappears.
- Take the test tubes out for cooling
- Note down the appearance of color seen in the test tubes.
Result and Interpretation of Iodine Test
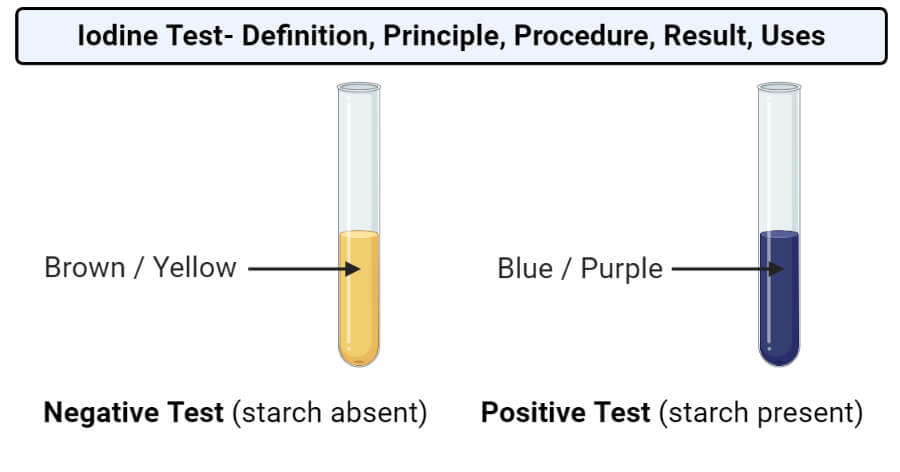
- The appearance of blue-black or purple color represents a positive test, indicating the presence of starch.
- If there is no change in color, the result is negative and indicates the absence of starch.
Uses of Iodine Test
- This test is used to detect the presence of starch in various samples.
- Similarly, this test is performed to test the process of photosynthesis in plants.
Limitations of Iodine Test
- This test cannot be performed under acidic conditions as the starch hydrolyses under such circumstances.
- This test is a qualitative test and doesn’t signify the concentration of starch.
References and Sources
- Tiwari A. (2015). Practical Biochemistry. LAP Lambert Academic Publishing.
- 2% – https://allmedicalstuff.com/barfoeds-test/
- 1% – https://www.scribd.com/document/429888468/Advances-in-Carbohydrate-pdf
- 1% – https://www.coursehero.com/file/p39m2q7c/IKI-Test-experiments-the-presence-of-starch-in-a-solution-if-positive-color/
- 1% – https://libraryofessays.com/lab-report/lap-report-1895512
- <1% – https://allmedtests.com/iodine-test-starch/

I hope to find out what type of Iodine I would need to just simply and correctly perform a starch test for detecting the presence of starches in my vitamin D3 supplement. Does it need to be using something like the Lugol’s iodine: 5% elemental iodine is mixed with 10% potassium iodide? Or does a basic Iodine solution from the drug store work with the 40% alcohol, and water already in it? Thank you for any info on this!
I believe you meant to say amylose instead of amylase in this sentence “Polyiodide ions form colored adsorption complex with helical chains of glucose residue of amylase (blue-black), dextrin (black), or glycogen (reddish-brown). Monosaccharides, disaccharides, and branched polysaccharides like cellulose remain colorless”
Hi Alina,
Yes, we meant to say “Amylose”. Thanks for the correction. The page has been updated. 🙂
would you get the same results if the starch solution was first heated then cooled and the drops of iodine solution was added
Very useful and understandable
Tnx for the observation
Hi
Thanx so much for the research
Please give a range of colours for negative tests
excellent,well spelt out and well arranged making it easier to understand,,Bingo????
Why colour disappeared on the addition of NaOH?
kind i may beseech you tutors to include the chemical reactions that those polysaccharides undergoes to give positive test for them
what is the reason of color disappearance in extra heat?
My biology teacher said the interactions between the iodine and starch are less tight or intense when it is heated, so the color is less intense. When it is cold, the lack of thermic energy makes the atoms closer and the coloration more intense. It goes both ways.
I would love to know what happened there in the reaction
Very helpful. I learnt more than I expected
Thank you a lot. Explained clearly and to the point.
So impactfully
It’s so useful for students..ilike it ????