Bacteriocins, small cationic molecules of about 30–60 amino acids, are proteinaceous compounds produced by lactic acid bacteria (LAB) having a bactericidal mode of action that can kill closely related bacteria.
- In 1928, the prototype LAB bacteriocin, nisin, was first developed.
- Bacteriocins form amphiphilic helices and become stable at 100 °C for 10 min. They vary in the spectrum of activity, molecular weight (MW), origin of genetics, mode of action, and biochemical properties.
- LAB acts as a natural bio-preservative. It shows antimicrobial activities that can produce various metabolites, including lactic acid, hydrogen peroxide, and bacteriocins.
- It has been used in food industries for fermenting food, such as for flavor and texture, and inhibiting the growth of spoilage and pathogenic microorganisms.
- By the expression of a specific immunity protein encoded in the bacteriocin operon, LAB strains producing bacteriocin prevent themselves from toxins produced by bacteriocin.
Interesting Science Videos
Class of LAB Bacteriocins
According to Klaenhammer in 1993, based on their primary structures, molecular weights, post-translational modifications, and genetic characteristics, LAB bacteriocins are classified into four classes.
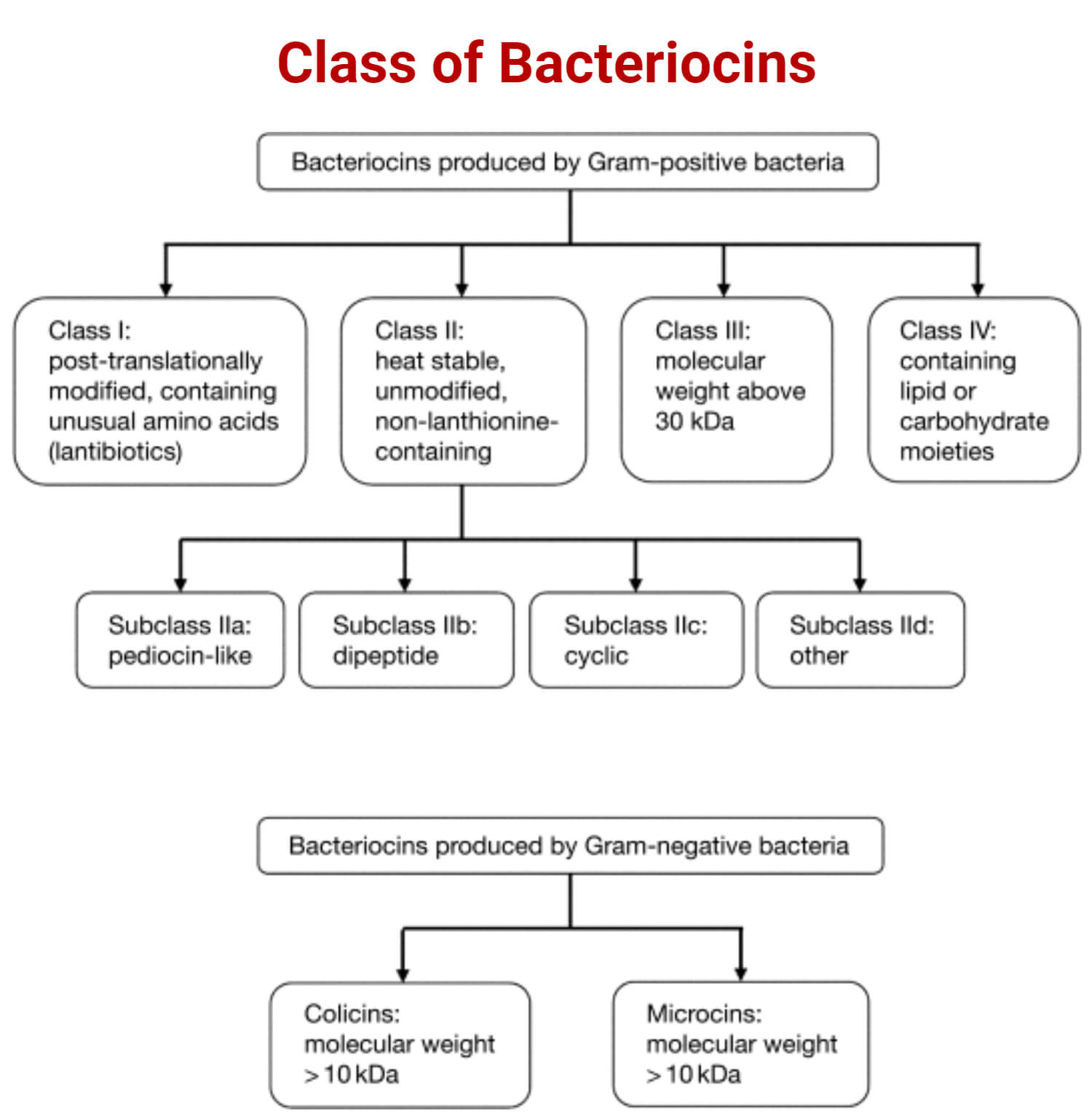
They are:
- Class I bacteriocins
- Class II bacteriocins
- Class III bacteriocins
- Class IV bacteriocins
Class I bacteriocins
- Class I bacteriocins are called lantibiotics. They are small (<5 kDa) peptides containing unique amino acids such as lanthionine (Lan) and β-methyllanthionine (MeLan).
- It consists of some dehydrated amino acids.
- Examples are nisin, lacticin, and mersacidin. Cytolysin and lacticin are the two-component lantibiotics. Enterococcus faecalis produces cytolysin and Lactobacillus lactis subsp. lactis produces lacticin 3147. Staphylococcus aureus produces staphylococcin C55
Class II bacteriocins
- Class II bacteriocins are small (<10 kDa), heat-stable, non-modified, cationic, hydrophobic, and non-Lan-containing membrane-active peptides.
- This class is classified into Class IIa, Class IIb, and Class IIc.
Class IIa
- Class IIa contains a double–glycine leader peptide and pediocin-like Listeria active peptides with the N-terminal consensus sequence YGNGV (Subclass IIa).
- Examples are pediocin PA1, sakicin A, enterocin A and leucocin A. It is now more inclined toward food preservation, with pediocin PA1 and leucocin.
- Source: Class IIa bacteriocins isolated from fermented meat, fermented vegetables, dairy products, smoked salmon, and the human gastrointestinal tract. Examples are Leuconostoc gelidum.
Class IIb
- Amphiphilic and hydrophobic regions are present in Class IIb bacteriocins, which are cationic.
- It consists of synergies of two complementary peptides. Examples are lactococcin G, lacticin F, plantaricin A, and enterocin X. The class IIb components are produced by Enterococcus faecium.
Class IIc
- Class IIc, the sec-dependent secreted bacteriocins, affects membrane permeability and cell wall formation.
- Examples are acidocin B, entereocin P, and reuterin 6. The class IIc components producing species are Lactobacillus acidophilus.
Class III bacteriocins
- Members of Class III are large molecular mass peptides (>30 kDa) and are heat-labile proteins. Examples are lysostaphin, enterolysin A, and helveticin J.
- The class III component-producing species is Lactobacillus helveticus, which produces helveticin J.
Class IV bacteriocins
- Class IV bacteriocins are complex types of bacteriocins that require large complexes of proteins with other macromolecules and non-proteinaceous moieties for activity.
- This class has not been studied much yet at the biochemical level.
What are Lantibiotics?
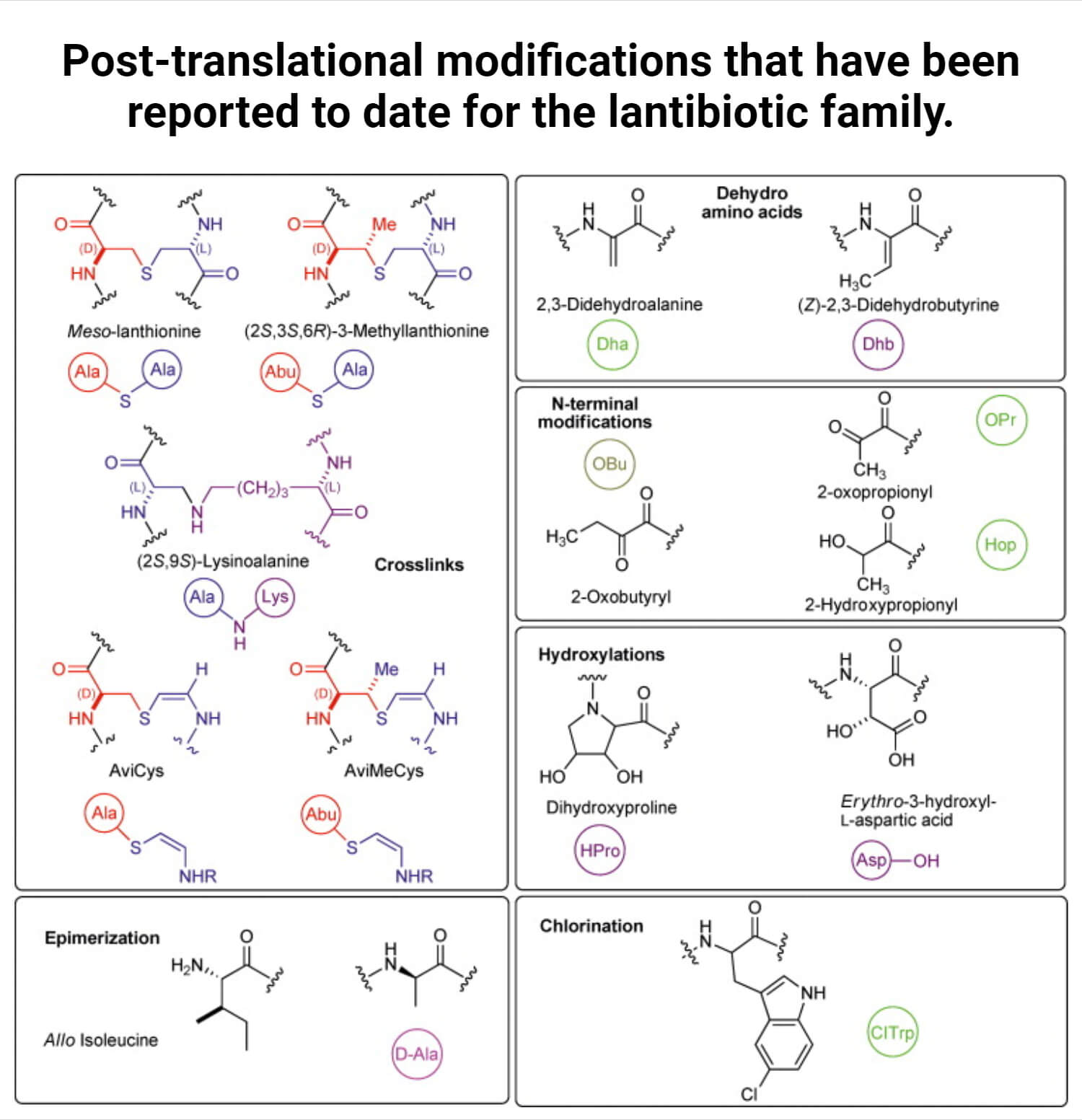
Lantibiotics, a type of bacteriocin, are synthesized ribosomally and post-translationally modified linear peptides. It has antimicrobial activity due to the presence of meso-lanthionine and 3-methyl-lanthionine.
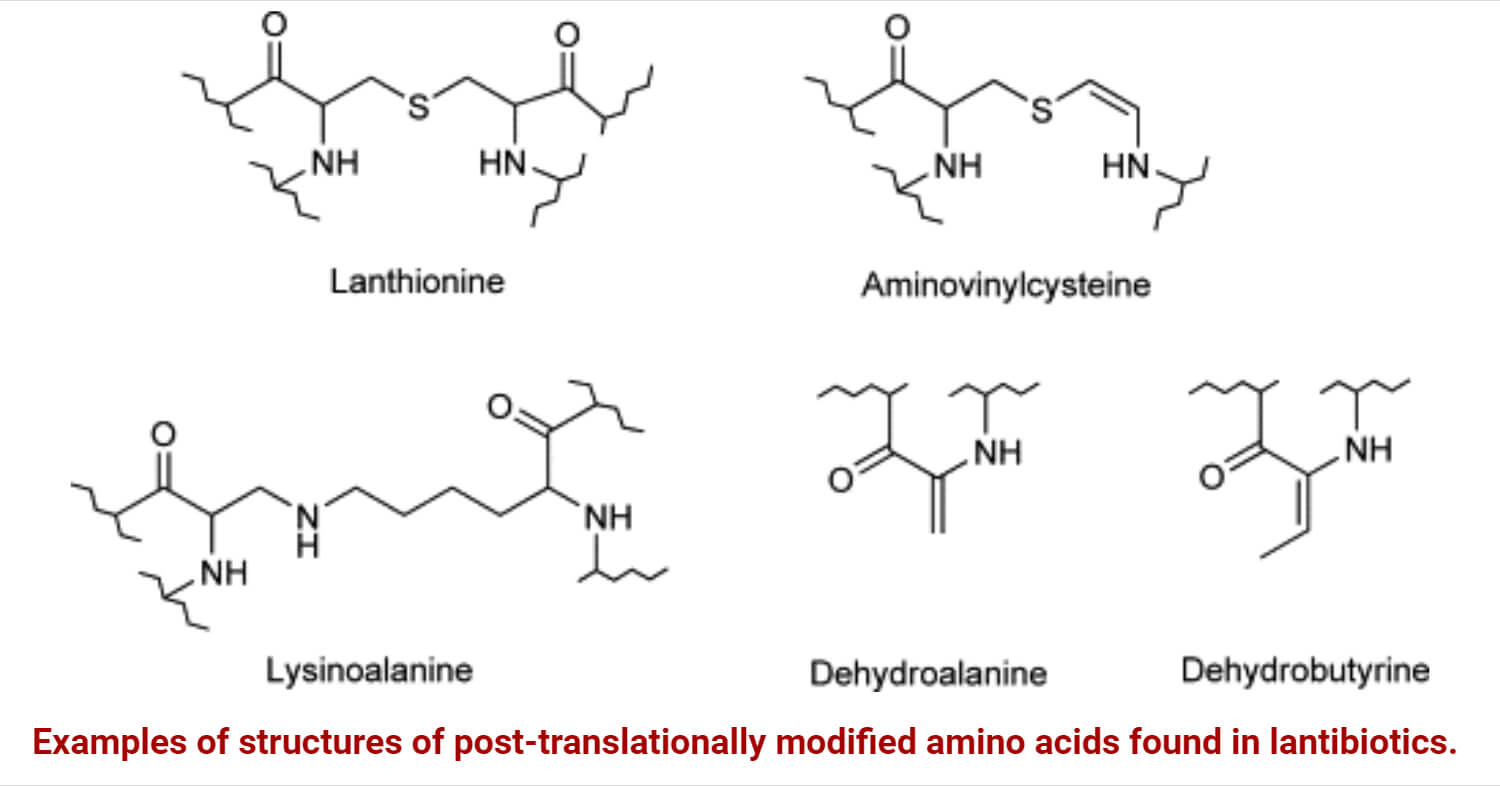
- Lantibiotics, lanthionine (lan)-containing antibiotic peptides, were first described in 1988.
- It consists of two alanine residues that connect at their -carbons by a thioether bridge. Moreover, they are incorporated within cyclic peptides.
- Lantibiotics, peptide-derived antimicrobial agents, are composed of unique amino acids, such as lanthionine (lan) and methyllanthionine (melan), so-called (lan)-containing antibiotics.
- It also contains dehydrated amino acids, D-amino acids, α-keto amides, and S-aminovinylcysteine.
- Lantibiotics produced by lactic acid bacteria are generally recognized as safe (GRAS), which is not harmful to human health. It has been in use for food safety and clinical applications.
- Gram-positive bacteria including the lactic acid bacteria (LAB), Bacillus, Enterococcus, Micrococcus, Streptococcus, Staphylococcus, and Actinomycetes produce lantibiotics that target other bacterial species during defense strategies.
- Before producing mature functional lantipeptide, it undergoes extensive post-translational modifications.
- Most lanthionine-containing RiPPs are lantibiotics that lack antimicrobial activity, referred to as lantipeptides.
- Lantibiotics are active against various food spoilage organisms, such as Listeria monocytogenes and Clostridium botulinum. In addition, It explicits promising activity against resistant Staphylococcus aureus and enterococcal infections.
Structural Aspects of Lantibiotics
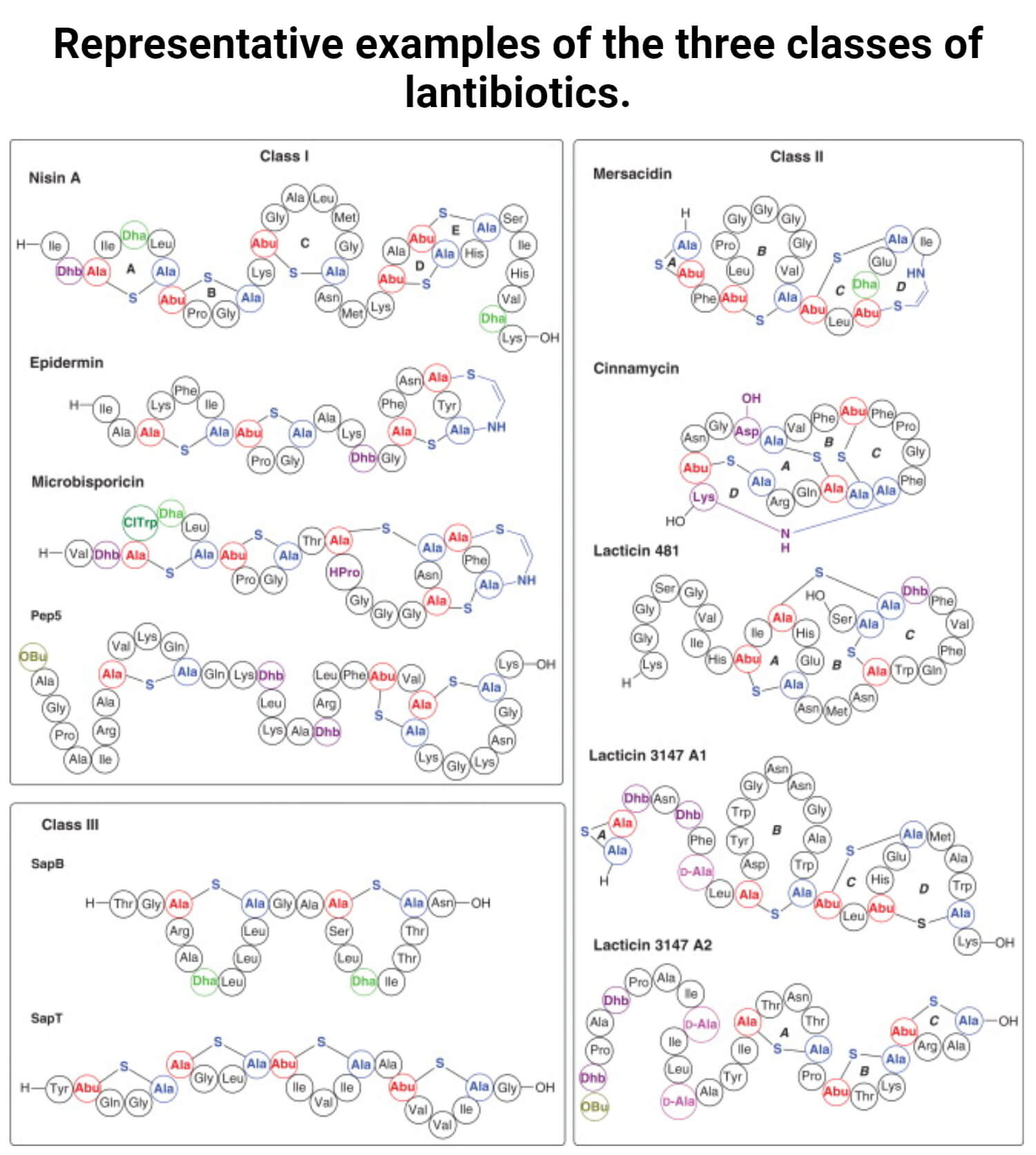
Based on their structural and functional features, type-A and type-B peptides are a group of antibiotics.
Type-A lantibiotics
- Type-A lantibiotics are elongated and cationic peptides up to 34 residues in length. It shows similarities in the arrangement of their Lan bridges.
- This peptide acts primarily in the membrane integrity of target organisms disrupts that includes nisin (the most-studied lantibiotic produced by Lactococcus lactis), subtilin, and epidermin.
Type-B lantibiotics
- Type-B lantibiotics are globular and have peptides up to 19 residues in length.
- These peptides act by disrupting the enzyme function, such as inhibiting the cell wall biosynthesis.
- The duramycins produced by Streptomyces species, and mersacidin produced by Bacillus species that are active against several Gram-positive bacteria include- methicillin-resistant S. aureus and actagardine, which are examples of type-B antibiotics.
Mode of action of Lantibiotics
- Lantibiotics have various modes of action to destroy bacteria. The inhibition of bacterial growth occurs by peptides’ ability to attach to lipid II and interfere with the bacterial cell wall’s production.
- In the cell membrane, It can form pores, which results in the loss of cellular contents and cell death.
- As a result of this dual mode of action, bacteria find it far more challenging to build functional resistance against lantibiotic peptides.
- When used independently, two-component lantibiotics may only have one of these modes of action (binding to lipid II).
- But when used in combination, they may have both modes of action (lipid II binding and pore creation).
Class of Lantibiotics
Based on their biosynthetic pathways, Lantibiotics are divided into four classes.
- Class I lantibiotics
The two various enzymes, LanB (dehydratase) and LanC (cyclase) modify Class I lantibiotics.
- Class II lantibiotics
A single LanM enzyme with dehydratase and cyclase activity modifies Class II lantibiotics.
- Class III and Class IV lantibiotics
A single enzyme- LanKC- modifies class III, whereas LanL modifies class IV. In addition, Class III possesses anti-allodynic/antinociceptive activity, antiviral activity, or morphogenetic activities.
Examples of Lantibiotics
| Lantibiotics | Producing Strains |
| Type-A Lantibiotics | |
| Type-A(I)- Nisin A | L. lactis NIZOR5, 6F3, NCFB894, ATCC11454 |
| Nisin Z | L. lactis N8, NIZO22186 |
| Subtilin | B. subtilis ATCC6633 |
| Epidermin | Staphylococcus epidermidis Tu3298 |
| Gallidermin | Staphylococcus gallinarum Tu3928 |
| Mutacin B-Ny266 | S. mutans |
| Mutacin 1140 | S. mutans JH1000 |
| Pep5 | S. epidermidis 5 |
| Epicidin 280 | S. epidermidis BN280 |
| Epilancin K7 | S. epidermidis K7 |
| Type-A (II) – Lacticin 481 – Cytolysin | L. lactis CNRZ481, ADRIA85LO30 E. faecalis DS16 |
| Lacticin 3147 | L. lactis DPC3147 |
| Staphylococcin C55 | S. aureus C55 |
| Salvaricin A | Streptococcus salvarius 20P3 |
| Lactocin S | L. sake L45 |
| Streptococcin A-FF2 | Streptococcus pyogenes FF22 |
| Sublancin 168 | B. subtilis 168 |
| Carnocin U149 | C. pisicola |
| Variacin 8 | Micrococcus varians MCV8 |
| Cypemycin | Streptomyces ssp. |
| Type-B antibiotics – Cinnamycin – Duramycin B | Streptomyces cinnamoneus Streptoverticillium ssp. |
| Duramycin C | Streptomyces griseoluteus |
| Ancovenin | Streptomyces ssp. |
| Mersacidin | B. subtilis HIL Y-85, 54728 |
| Actagardine | Actinoplanes |
Applications of Lantibiotics
- Lantibiotics are at the center of attraction in food preservation and safety. Examples: Nisin has been used extensively as a preservative against foodborne pathogens.
- It can be used in the field of human and animal therapeutics.
- It has been used in various potential medical applications and some in clinical trials.
- It helps to improve their in vivo efficacy under various conditions, such as stroke and diabetic nephropathy.
References
- Olivia McAuliffe, R. Paul Ross, Colin Hill, Lantibiotics: structure, biosynthesis and mode of action, FEMS Microbiology Reviews, Volume 25, Issue 3, May 2001, Pages 285–308, https://doi.org/10.1111/j.1574-6976.2001.tb00579.x
- Field, D., Cotter, P. D., Hill, C., & Ross, R. P. (2015). Bioengineering Lantibiotics for Therapeutic Success. Frontiers in microbiology, 6, 1363. https://doi.org/10.3389/fmicb.2015.01363
- Ross, A., Vederas, J. Fundamental functionality: recent developments in understanding the structure–activity relationships of lantibiotic peptides. J Antibiot 64, 27–34 (2011). https://doi.org/10.1038/ja.2010.136
- Mokoena M. P. (2017). Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules (Basel, Switzerland), 22(8), 1255. https://doi.org/10.3390/molecules22081255
- Olivia McAuliffe, R. Paul Ross, Colin Hill, Lantibiotics: structure, biosynthesis and mode of action, FEMS Microbiology Reviews, Volume 25, Issue 3, May 2001, Pages 285–308, https://doi.org/10.1111/j.1574-6976.2001.tb00579.x
- van Staden, A. D. P., van Zyl, W. F., Trindade, M., Dicks, L. M. T., & Smith, C. (2021). Therapeutic Application of Lantibiotics and Other Lanthipeptides: Old and New Findings. Applied and environmental microbiology, 87(14), e0018621. https://doi.org/10.1128/AEM.00186-21
- Chakraborty, H.J., Gangopadhyay, A. & Datta, A. Prediction and characterisation of lantibiotic structures with molecular modelling and molecular dynamics simulations. Sci Rep 9, 7169 (2019). https://doi.org/10.1038/s41598-019-42963-8
