Biuret Test is the test used to detect the presence of peptide bonds in the sample and to test for the presence of proteins or peptides.
Proteins and peptides are polymers of amino acids. They are chains of amino acids as well as other biomolecules or ions or compounds. The amino acids are covalently bound to each other by a covalent bond, called a peptide bond, between the carbon number one (C1) of one amino acid and nitrogen number two (N2) of adjacent amino acid. The formation of a peptide bond is a condensation reaction.
In the process carboxylic acid moiety of one amino acid loose hydrogen and oxygen, the amino moiety of another amino acid loses hydrogen and the exposed carbon of the 1st amino acid and the exposed nitrogen of the 2nd amino acid join to form a dipeptide with a peptide bond (-CO-NH-).
The nitrogen atom in a peptide bond of proteins and peptides contains unshared electrons. These unshared electrons of the peptide bonds, in an alkaline environment, can be used by cupric ion (Cu+2) present in the Biuret reagent to form a violet or purple-colored complex.
This colorimetric chemical test used to detect the peptide bond using the Biuret reagent is called the Biuret test. It is also called Piotrowski’s reaction after the name of the Polish physiologist Gustaw Piotrowski who observed this phenomenon in 1857 and used it to detect proteins in samples.
In the Biuret reagent, the compound Biuret is not actually used. Biuret is a chemical compound having a molecular formula of HN(CONH2)2 which is formed by the condensation of two urea molecules when urea is heated at 150°C. A similar reaction producing a purple-colored complex compound was first noted when biuret reacts with Cu+2 ions because biuret has bonds similar to peptide bonds. Hence the test is named Biuret test due to the similarity in the end products.
It is used in labs to detect the presence of peptides or proteins in a sample. It is a qualitative test, and can only state the presence or absence of the peptide bonds but demonstrate nothing about the exact quantity and type of proteins.
Interesting Science Videos
Objectives of Biuret Test
- To detect the presence of peptide bonds in the sample.
- To test for the presence of proteins or peptides.
Principle of Biuret Test
The reaction in the biuret test is a colorimetric reaction where the result is indicated by a color change from blue to purple or violet. In an alkaline environment, the cupric (Cu+2) ions in the biuret reagent bind to the nitrogen atoms in the peptide bonds of proteins forming a violet-colored copper coordination complex. The formation of purple color indicates the presence of peptide bonds in the sample. The intensity of the developed purple color is directly proportional to the concentration of peptide bonds present in the solution.
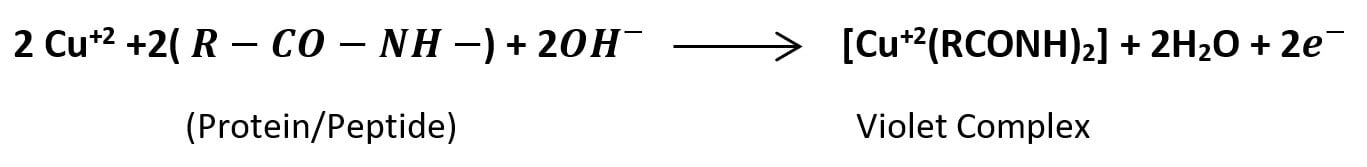
Requirements for Biuret Test
Biuret Reagent
Alkaline copper sulfate (CuSO4) solution is used as the Biuret reagent. It is blue in color due to the color of CuSO4.
Components of Biuret Reagent
- Copper sulfate (CuSO4) solution
- Sodium potassium tartrate
- Sodium hydroxide (NaOH) or potassium hydroxide (KOH) solution
- Distilled water
Preparation of Biuret Reagent
- Dissolve 1 gram of CuSO4 crystals in 100 mL of distilled water.
- Add 1.2 grams of sodium potassium tartrate to the mixture. (it stabilizes the Cu+2 ions)
- Dissolve 10 grams of NaOH pellet in 90 mL of distilled water to make a 10% NaOH solution.
- Add 10 mL of the 10% NaOH solution to 100 mL of 1% CuSO4 solution.
Equipment
Test tubes Dropper Test tube stand
PPE and other general laboratory equipment
Samples (Test solution)
- Positive Control: Albumin (protein or test solution) solution
- Negative Control: Plane water (Distilled water or sugar solution)
Procedure of Biuret Test
- Label three test tubes as ‘test’, ‘positive’, and ‘negative’.
- In the test tube labeled as ‘test’, dispense 1-2 mL of sample, in the test tube labeled as ‘positive’, dispense 1-2 mL of albumin solution, and in the test tube labeled as ‘negative’, dispense 1-2 mL of distilled water.
- In each tube, add an equal volume of (1-2 mL) of Biuret reagent.
- Shake well and let it stand at room temperature for 5 minutes.
- Observe the tubes for the development of violet color in the suspension.
Result and Interpretation of Biuret Test
- Positive Biuret Test: Formation of purple color after the addition of Biuret reagent. (Tube with albumin solution will turn purple.)
- Negative Biuret Test: No formation of violet/purple color (or formation of blue color) solution after the addition of Biuret reagent. (Water will turn to blue color.)
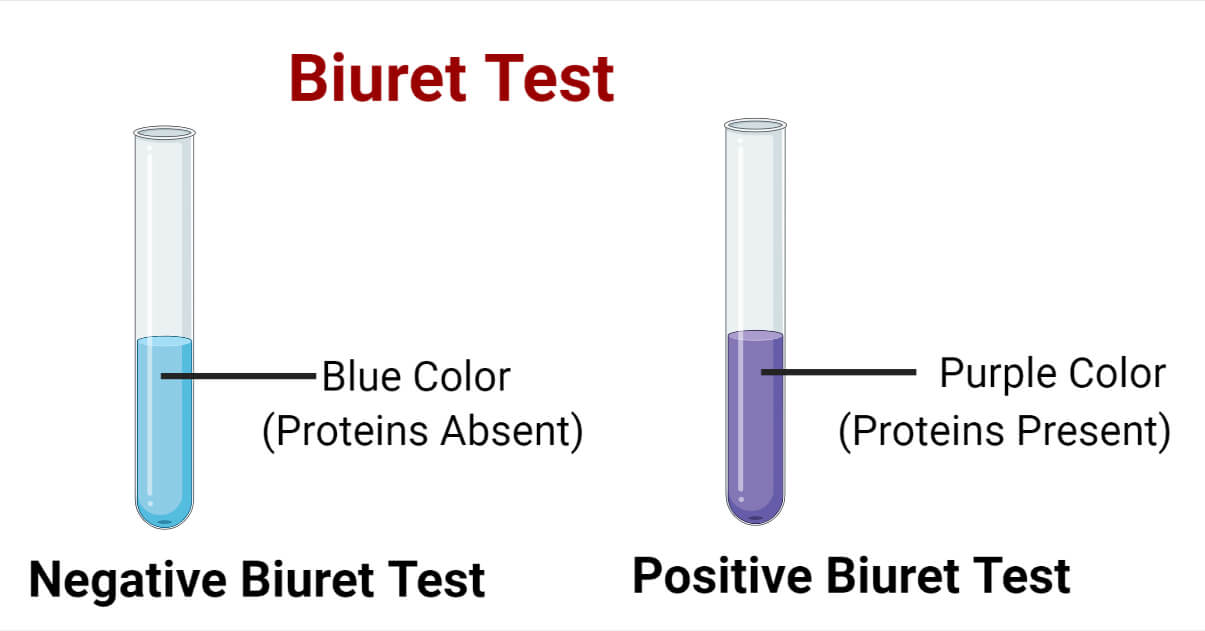
Accordingly, if the color of the sample solution turns to violet/purple after the addition of the Biuret reagent and incubation, report the sample positive for proteins/peptides.
If the color of the sample doesn’t change i.e. remains blue even after 5 minutes of the addition of Biuret reagent, report the sample negative for proteins/peptides.
Precautions
- Use the proper amount of sample and reagent; generally, the 1:1 ratio gives a better result.
- Excessive use of reagent will form the mixture blue instead of purple giving a false negative result.
- Don’t read the result before 3-5 minutes. You may get a false negative result.
Applications of Biuret Test
- Detection of proteins in any unknown solution or extracts.
- Detection of proteins in urine, CSF, and other body fluids.
- Used in food analysis to detect the addition of proteinaceous adulterants in non-protein products.
- Used in biotechnology and biochemistry research purposes.
Limitations of Biuret Test
- We can’t exactly quantify the number of proteins present in the sample.
- Only soluble proteins can be detected.
- Ammonium and magnesium ions, carbohydrates, fats, and turbidity can hinder the reaction.
- Amino acid histidine also gives a positive result.
References
- Biuret Test for Protein- Definition, Principle, Procedure, Results, Uses (biochemden.com)
- What does Biuret test for? Its Principle, Mechanism and Uses – Laboratoryinfo.com
- Biuret Test – Principle, Preparation and Procedure (vedantu.com)
- Biuret Test: Principle, Reagent, Procedure &Result Interpretation – BIOCHEMINSIDER
- Biuret test: Principle, Requirements, Procedure and Result Interpretation – Online Science Notes
- Biuret Test Lab Report – 897 Words | Internet Public Library (ipl.org)
- Biuret Test – Checking for Peptide Bonds with Biuret Reagent (byjus.com)
- Biuret Test: Definition, Theory, Procedure, and Results (chemistrylearner.com)
- Biuret test: Principle, Reaction, Requirements, Procedure and Result Interpretation | Online Biochemistry Notes (biocheminfo.com)
- Biuret test. (2022, December 6). In Wikipedia. https://en.wikipedia.org/wiki/Biuret_test

“What an insightful guide to the Biuret Test for Protein! This comprehensive resource covers everything from the principle and procedure to results and practical applications. A must-read for anyone delving into the fascinating world of protein analysis. Thank you for sharing this valuable information!” The Indian Electrician .
Wonderful 💪💪
You guys are indeed the best so far.
I am impressed
Thenks for your answer because May make me having the good understanding 🙏🇹🇿
Wow wow wow 💓💓💓💓💓💓 I really love the presentation. The report has really helped me. And I’m sure from now on, writing these lab report won’t be a problem.
Keep up the good work 💪💪🇿🇲
Wow wow what a nice report keep it up👊👊👊👊
How be learning from this site😎
✔️✔️✔️✔️✔️✔️
Hello!! Thank you for your answer Am wishing good Improvement
I recently saw a video which shows that tap water is giving a positive test with the foviself kit. Can this be explained .why should tap water give a positive (protein ?) test?
i like it
Please i will like to be learning from this site.