Bacillus subtilis is the type species of the genus Bacillus which is commonly found in diverse environments ranging from soil to the gastrointestinal tract of cattle and humans.
It is a Gram-positive, rod-shaped, spore-forming, and facultative anaerobe that is the most commonly isolated Bacillus species from environmental samples.
B. subtilis has been extensively studied as a model for cell differentiation and engineering in biotechnology. It is also known as hay Bacillus or grass Bacillus as it is widespread in different types of grasses and hay sources.
B. subtilis is the most studied Gram-positive bacterium as it is studied as a model organism for studies regarding bacterial chromosome replication and transformation. It is ubiquitous in distribution which is facilitated by the resistance of the bacteria to cold, heat, and common disinfectants.
Most B. subtilis species are non-pathogenic and are not associated with infections, but some strains have been associated with neoplastic diseases like fatal pneumonia and bacteremia, septicemia, and infections of necrotic axillary tumors in breast cancer. Some strains have also been implicated in foodborne illness and cases of bovine mastitis and ovine abortion.
B. subtilis was first isolated in 1835 by Ehernberg who also named the bacterium, Vibrio subtilis, but it was later reclassified and named by Cohn in 1872. The species name ‘subtilis’ is a Latin word that means ‘slender’ indicating the long rod-shaped structure of the bacteria.
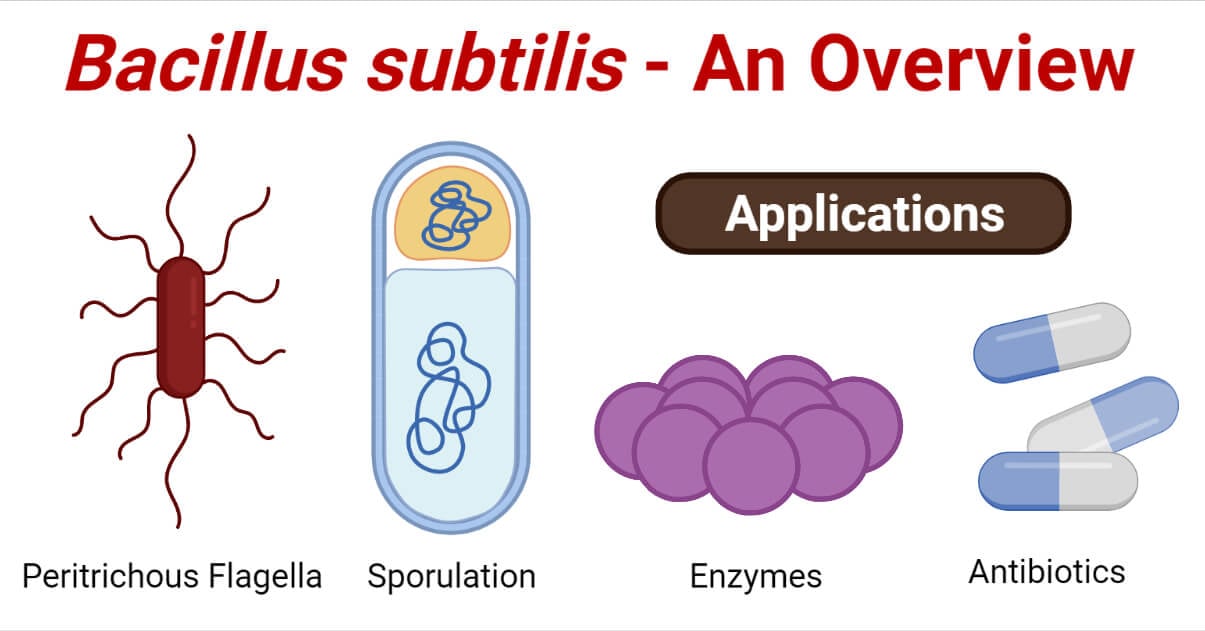
B. subtilis is considered an essential industrial bacterium as it is widely used in biotechnology due to the improved expression and secretion of enzymes by the bacteria. Besides, it is also used in food industries as flavor enhancers, sweeteners, and animal feed. B. subtilis are further classified into two subspecies; Bacillus subtilis subsp. subtilis and Bacillus subtilis subsp. spizizenii.
Interesting Science Videos
Classification of Bacillus subtilis
- The genus Bacillus belongs to the family Bacillaceae on the basis of phylogenetic analysis of 16S rRNA gene sequences.
- The family consists of Bacillus and 18 other genera, the majority of which are aerobic or facultatively anaerobic chemoorganotrophs.
- The genus Bacillus consists of more than 100 different species which are grouped into manageable and better-defined groups.
- The initial classification of Bacillus species was based on phenotypic, cultural, and metabolic characteristics of the bacteria.
- The grouping of species is based on the similarity of the 16S rRNA gene sequences and DNA-DNA hybridization.
- B. subtilis belongs to group 1 of Bacillus, and it is closely related to other species like B. licheniformis and the pathogenic group of Bacillus species including B. cereus, B. anthracis, and B. thuringiensis.
- Group 1 of Bacillus species consists of different species that are industrially important for the production of different compounds.
- The B. subtilis species are further classified into two subspecies; B. subtilis subsp. subtilis and Bacillus subtilis subsp. spizizenii. These subspecies cannot be distinguished on the basis of phenotypic characteristics and require genotypic analysis.
- The following is the taxonomical classification of B. subtilis:
| Domain | Bacteria |
| Phylum | Firmicutes |
| Class | Bacilli |
| Order | Bacillales |
| Family | Bacillaceae |
| Genus | Bacillus |
| Species | B. subtilis |
Habitat of Bacillus subtilis
- Bacillus is ubiquitous in distribution and is found in various habitats throughout the world, ranging from soil to human and animal bodies.
- Most aerobic endospore-forming bacteria like Bacillus are saprophytes that are distributed in the natural environments in the form of spores.
- However, some might be found in animate surfaces like tissues as opportunistic or obligate pathogens.
- The most important habitat of B. subtilis is soils of different kinds, ranging from acid to alkaline, cold to hot, and fertile to the desert. The types of strains living in the habitats depend on the water content and deposits.
- The availability of B. subtilis in different environments is due to the distribution of bacterial spores in the form of aerosols.
- Besides, B. subtilis can survive in a wide range of different temperatures from 15°C to 55°C.
- Some variety of B. subtilis can be found as ubiquitous contaminants of food, water, and environments that are natural, domestic, industrial, and hospital.
- The endospores formed by B. subtilis are resistant to physical and chemical agents like temperature, disinfectants, antibiotics, and toxic compounds.
- Bacterial spores are also found in high concentrations in dried foods like spices, milk powder, and other products.
- These spores are dispersed easily by the wind, which allows the spores to migrate to long distances and discover new ecological niches.
- B. subtilis are heterotrophic organisms that are isolated from environments with complex nutrient availability and environmental conditions.
- The occurrence of B. subtilis in soil and the rhizospheric area might exist in a close relationship with the plants by helping in the production of phytohormones and enhancement of root nodulation.
- Some spores of B. subtilis can also be found in animal surfaces like the human intestinal tract and skin surfaces and can be isolated from samples like human feces.
Morphology of Bacillus subtilis
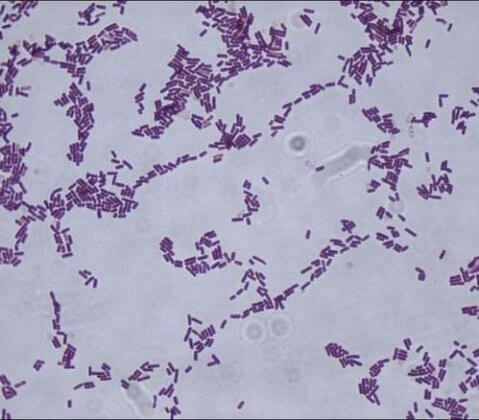
- Bacillus subtilis is a Gram-positive, rod-shaped bacterium that is the type species of the genus Bacillus, commonly used as a model organism to describe the structure of different species of the genus.
- The cells of B. subtilis are Gram-positive motile rods that form ellipsoidal to cylindrical spores present centrally or paracentrally in the swollen sporangia. The spores are visible inside the dormant cell via spore staining.
- A single endospore is present in a cell, and these spores are very resistant to adverse environmental conditions.
- The cells are round-ended and vary in size ranging between 0.7-0.8 µm × 2.0-3.0 µm.
- The arrangement of the cells is mostly single or in pairs; B. subtilis rarely form chains. The cells are motile with peritrichous flagella.
- Even though B. subtilis was initially considered obligate aerobe, but based on more recent findings, these are known to be facultative anaerobe.
- Most strains of B. subtilis are non-capsulated, but some strains produce capsules composed of polyglutamic acid or polysaccharides.
- The production of poly-γ-glutamic acid by B. subtilis occurs during the stationary phase of growth.
- The cell wall of B. subtilis cells is composed of peptidoglycan units with the most common type of linkage being meso-diaminopimelic acid.
- The cross-linkage is formed in the form of a peptide bond between the diamino acid in position 3 of one subunit and the D-alanine in position 4 of the neighboring peptide subunit.
- Underneath the cell wall is a cell membrane that is made up of lipid bilayer and protein structures that determine the fluidity of the membrane.
- The cytoplasm consists of a circular chromosome, mitochondria, and chloroplasts distributed throughout the cell.
- Filament-forming proteins are present along the longer axis of the cell that pushes the newly replicated DNA after cell division.
- The genome of B. subtilis is 4214810 bp long with about 4000 protein-coding genes. The G+ C content of the bacteria ranges between 40-45%.
Cultural Characteristics of Bacillus subtilis
- The colony morphologies of B. subtilis are highly variable, within and between strains which may give the appearance of a mixed culture during growth on an artificial medium.
- In spite of the diversity, the colonies of Bacillus species can be recognized on agar plates quite easily.
- The growth on a simple medium like nutrient agar might result in the swarming growth of the bacteria through the plate. This can be avoided by increasing the agar content of the media.
- Bacillus species usually have simple nutrient requirements which allow their growth in simple non-selective media like Nutrient Agar.
- The optimum temperature for the growth of B. subtilis is 28-30°C with a minimum temperature of 5-20°C and a maximum temperature of 45-55°C.
- B. subtilis isolates from food samples optimally at 20-25°C, whereas that from clinical samples grow well at 35°C.
- Growth of B. subtilis can be seen within the pH range of 5.5 to 8.5, but the growth of some strains might be limited even within the said range.
- The growth of B. subtilis can occur on a minimal medium with glucose and ammonium salt as the sole sources of carbon and nitrogen, respectively.
- Most of the strains can tolerate 7% NaCl in the medium, but some can tolerate up to 10% NaCl.
- Even though B. subtilis are known as obligate aerobes; some restricted growth can be observed under anaerobic conditions in complex media with glucose or even nitrate.
- In liquid culture, LB broth is commonly used for the culture of B. subtilis. The growth is observed in the form of turbidity, and the cells begin to settle down as the growth ceases.
- The following are some cultural characteristics of B. subtilis in different culture media:
1. Bacillus subtilis in Nutrient Agar
- The colonies of B. subtilis on nutrient agar are round to irregular in shape. The isolates obtained from soil samples tend to form swarming growth throughout the plate.
- The size of the colonies is also variable ranging between 2-3 mm in diameter as the younger cultures tend to be larger and older colonies shrink up in size.
- The colonies have varying margins varying from undulate to fimbriate. The colonies are opaque with surfaces that are dull or even wrinkled.
- The color of the colonies is mostly white but can range between creamy and brown. Some strains produce varying pigments like creamy, yellow, orange, pink, and red to brown and black depending on the source or sample.
- These pigments are often observed in potato agar or glucose-containing agar medium.
- Strains that produce brown or black pigments were formerly called Bacillus subtilis var aterrimus, whereas those producing brownish-black pigment on tyrosine-containing media were name B. subtilis var niger.
2. Bacillus subtilis in Blood Agar
- B. subtilis form grey or white-colored colonies that are round, opaque, flat, and dry on blood agar supplemented with 5% rabbit blood.
- The colonies are medium-sized (ranging between 3-4 mm in diameter) that often dry on the surface as the culture dries out.
- Most strains of B. subtilis show β-hemolysis in the form of clearing of the media with the hemolysis of red blood cells. This is more common in B. subtilis obtained from clinical samples than from environmental samples.
3. Bacillus subtilis in Tryptic Soy Agar
- White to creamy colored colonies of B. subtilis are obtained on Tryptic Soy Agar. The colonies are circular or irregular in shape depending on the strain and the conditions for growth.
- The colonies have an irregular margin, and they are mostly flat. The surface is opaque and mucoid.
- On this agar, optimal growth occurs at 35°C under aerobic conditions. Some species may be facultatively anaerobic and might grow better in some 2% CO2.
Biochemical Characteristics of Bacillus subtilis
The biochemical characteristics of B. subtilis can be tabulated as follows:
| S.N | Biochemical Characteristics | Bacillus subtilis |
| 1. | Capsule | Most strains are non-capsulated, but some might contain a polyglutamic capsule. |
| 2. | Shape | Rod |
| 3. | Gram Staining | Gram-Positive |
| 4. | Catalase | Positive (+) |
| 5. | Oxidase | Variable |
| 6. | Citrate | Positive (+) |
| 7. | Methyl Red (MR) | Negative (-) |
| 8. | Voges Proskauer (VR) | Positive (+) |
| 9. | OF (Oxidative-Fermentative) | Facultative Heterofermentative |
| 10. | Coagulase | Positive (+) |
| 11. | DNase | Negative (-) |
| 12. | Urease | Negative (-) |
| 13. | Gas | Negative (-) |
| 14. | H2S | Negative (-) |
| 15. | Hemolysis | β-hemolytic |
| 16. | Motility | Motile with peritrichous flagella |
| 17. | Nitrate Reduction | Positive (+) |
| 18. | Gelatin Hydrolysis | Positive (+) |
| 19. | Pigment Production | Positive (+) |
| 20. | Indole | Negative (-) |
| 21. | TSIA (Triple Sugar Iron Agar) | Alkali/Alkali (Red/ Red) |
| 22. | Spore | Endospore-forming |
Fermentation
| S.N | Substrate | Bacillus subtilis |
| 1. | Adonitol | Negative (-) |
| 2. | Arabinose | Positive (+) |
| 3. | Cellobiose | Positive (+) |
| 4. | Dulcitol | Negative (-) |
| 5. | Fructose | Positive (+) |
| 6. | Galactose | Positive (+) |
| 7. | Glucose | Positive (+) Facultative heterofermentative |
| 8. | Glycerol | Positive (+) |
| 9. | Glycogen | Positive (+) |
| 10. | Hippurate | Negative (-) |
| 11. | Inulin | Positive (+) |
| 12. | Inositol | Positive (+) |
| 13. | Lactose | Positive (+) |
| 14. | Malonate | Positive (+) |
| 15. | Maltose | Positive (+) |
| 16. | Mannitol | Positive (+) |
| 17. | Mannose | Positive (+) |
| 18. | Melibiose | Variable |
| 19. | Pyruvate | Negative (-) |
| 20. | Raffinose | Positive (+) |
| 21. | Rhamnose | Negative (-) |
| 22. | Ribose | Positive (+) |
| 23. | Salicin | Positive (+) |
| 24. | Sorbitol | Positive (+) |
| 25. | Starch | Positive (+) |
| 26. | Sucrose | Positive (+) |
| 27. | Trehalose | Positive (+) |
| 28 | Xylose | Positive (+) |
Enzymatic Reactions
| S.N | Enzymes | Bacillus subtilis |
| 1. | Acetoin | Positive (+) |
| 2. | Acetate Utilization | Positive (+) |
| 3. | Tyrosine Hydrolysis | Negative (-) |
| 4. | Lecithinase | Negative (-) |
| 5. | Casein Hydrolysis | Positive (+) |
| 6. | Esculin Hydrolysis | Positive (+) |
| 7. | Lysine | Negative (-) |
| 8. | Ornithine Decarboxylase | Negative (-) |
| 9. | Phenylalanine Deaminase | Negative (-) |
B. subtilis can decompose pectin and polysaccharides of plant origin. Dextran and levan are formed extracellular from sucrose during carbohydrate degradation.
Virulence Factors of Bacillus subtilis
- Bacillus subtilis is ubiquitous in distribution and is found in different environmental regions like air, water, soil, and even animal and human body surfaces.
- The ability of the bacteria to bind and colonize different parts of the human and animal bodies indicates a possible virulence factor of the bacteria.
- However, different studies have indicated that the colonization of human body surfaces doesn’t involve a distinct mechanism that might be involved in disease production.
- A possible virulence factor of B. subtilis is toxin production as the bacteria produces the enzyme lecithinase which has been shown to be involved in food poisoning.
- Besides, B. subtilis also produces an extracellular toxin called subtilisin which is a proteinaceous compound capable of causing allergic reactions in some individuals.
- These reactions are often observed in immunocompromised individuals when they are exposed to such toxins regularly.
- The cases of allergies and hypersensitivity reactions, including dermatitis and respiratory distress, are often observed after the use of laundry products that are made with the said toxin.
- The virulence of B. subtilis, as well as B. subtilis toxins, is relatively low and it has been suggested that the bacteria do not produce significant quantities of the enzymes or toxins.
Role of Bacillus subtilis in animal and plant diseases
- Bacillus subtilis has been isolated from different cases of bovine and ovine abortions, but it hasn’t been implicated as the causative agent of such infections.
- Besides, B. subtilis has been associated with cases of bovine mastitis, but the number of cases of mastitis caused by B. subtilis is low when compared to other species.
- B. subtilis has also been shown to be capable of infecting and resulting in the death of 2nd instar larvae of the mosquito.
- The ability of B. subtilis to cause infections in insects indicates the potential of the use of B. subtilis as a biocontrol agent.
- B. subtilis is not considered a plant pathogen, but there have been some reports regarding the involvement of B. subtilis in the soft rot of garlic cloves.
- Based on a report, it was assumed that B. subtilis might be involved in the broad open cancer ulcrea in maple trees.
- The occurrence of B. subtilis in both animals and plants is quite limited and is not the primary causative agent.
Industrial uses / Applications of Bacillus subtilis
The use of Bacillus species as workhorse industrial microorganisms has been done for thousands of years with application in applied microbiology.
The high growth rates of the bacteria, short fermentation cycle, and the capacity to secrete extracellular proteins and enzymes enable the use of Bacillus subtilis in different industries.
B. subtilis is one of the few species of Bacillus that is considered “Generally Regarded as Safe” (GRAS) by the Food and Drug Administration.
The development and exploitation of B. subtilis are enabled by the information that is available on the biochemistry and genetics of the organisms.
The following are the industrial application of B. subtilis and its products:
1. Enzymes
- It has been estimated that Bacillus accounts for about 50% of the total industrially important enzymes in the world.
- Among all the species of Bacillus, B. subtilis is the most important species for industrial application.
- The alkaline serine proteases (subtilisins) produced by B. subtilis are primarily used in the production of household detergents.
- The alkalophilic organism and enzymes can be used in heavy-duty enzyme production with high alkaline tolerance.
- Besides, neutral B. subtilis produce proteases that are zinc metalloproteinases and have application in milk protein modification, nitrogen control, mash extraction and chill-haze removal in brewing industries.
- The amylases can be used in a number of industrial processes like food, fermentation, textile and paper industries.
- α-amylases cleave the internal α-1,4-linkages internally to produce shorter strands of carbohydrates that can then be used in different industries.
- Similarly, β-amylases are also industrially important as they operate to remove maltose units externally.
- B. subtilis also produces glucose isomerases that are essential in the final stages of starch processing to sweeteners. These enzymes are important for the conversion of glucose syrups to high fructose corn syrups.
- Other enzymes like cellulases, chitinases, and tannases also have industrial importance in the paper and textile industries.
- B. subtilis is one of the most potent producers of alkaline proteases that have an alkaline pH range and good thermostability. Alkaline proteases are used in the production of detergents and in abating, dehairing leather, and recovery of silver from X-ray films.
- Variants of B. subtilis enzymes have been prepared commercially through protein engineering to produce enzymes with improved performance.
2. Antibiotics
- Different species of B. subtilis produce various classes of antibiotics that are effective against different Gram-positive and Gram-negative bacteria. The compounds are produced during the early stages of sporulation.
- B. subtilis has also been used to transport bacitracin biosynthetic gene clusters from other Bacillus species like B. licheniformis to produce the compounds more efficiently.
- B. subtilis produces other antimicrobial compounds like subtilin, bacilysin, subsporins, lipooligopeptides, and rhizocticins.
- A lipopeptide antibiotic surfactin is also produced by B. subtilis which has potential antitumoral, antiviral, antibacterial, and hypocholesterolemic activity.
3. Purine nucleotides
- Purine nucleotides and nucleosides have applications in different industries like medicine and as flavor enhancers.
- B. subtilis produces nucleosides by subsequent chemical phosphorylation. In some cases, mutants of B. subtilis are used to produce inosine which easily passes through the cell membrane into the extracellular medium.
- Besides inosine, B. subtilis is also used for the production of other nucleotides like guanosine, riboflavin, and folic acid.
4. Vitamins
- Some strains of B. subtilis can be used for the limited production of some vitamins by fermentation.
- Processes like the cloning of riboflavin, cobalamin, and biotin biosynthesis are exploited in order to produce vitamins in a commercial way.
- In B. subtilis, deregulation of purine synthesis and a mutation in a flavokinase-flavin adenine dinucleotide synthetase is required to produce riboflavin from B. subtilis.
- Recombinant DNA techniques, along with fermentation strategies, are used to develop commercially important levels of riboflavin in B. subtilis.
5. Poly-γ-glutamic acid
- γ-polyglutamic acid is a naturally occurring anionic homopolyamide that is composed of D- and L-glutamic acid units connected by amide linkages.
- The acid is a water-soluble, edible and biodegradable compound with application in different industries like food, cosmetic and medical areas.
- Poly-γ-glutamic acid and its derivatives are used as thickeners, humectants, cryoprotectants, drug carrier and heavy metal absorber.
- Besides, it can also be used for wastewater treatment as a biopolymer flocculent as well as an animal feed additive.
6. D-Ribose
- D-Ribose is often used as a flavour enhancer in different industrial products like cosmetics, pharmaceuticals, food and animal feed.
- It also has application in the treatment of myocardial ischemia and muscular pain.
- Several strains of B. subtilis can produce D-ribose via fermentation which can be increased by applying genetic engineering technology.
References
- Topley WWC (2007). Topley and Wilson’s Microbiology and Microbial Interactions; Bacteriology, 2 Vol. Tenth Edition. John Wiley and Sons Ltd.
- Bergey, D. H., Whitman, W. B., De, V. P., Garrity, G. M., & Jones, D. (2009). Bergey’s manual of systematic bacteriology: Vol. 3. New York: Springer
- Earl, Ashlee M et al. “Ecology and genomics of Bacillus subtilis.” Trends in microbiology vol. 16,6 (2008): 269-75. doi:10.1016/j.tim.2008.03.004
- Amuguni, Hellen, and Saul Tzipori. “Bacillus subtilis: a temperature resistant and needle free delivery system of immunogens.” Human vaccines & immunotherapeutics vol. 8,7 (2012): 979-86. doi:10.4161/hv.20694
- Borriss, Rainer et al. “Bacillus subtilis, the model Gram-positive bacterium: 20 years of annotation refinement.” Microbial biotechnology vol. 11,1 (2018): 3-17. doi:10.1111/1751-7915.13043
- Gu, Han-Jie et al. “A First Study of the Virulence Potential of a Bacillus subtilis Isolate From Deep-Sea Hydrothermal Vent.” Frontiers in cellular and infection microbiology vol. 9 183. 31 May. 2019, doi:10.3389/fcimb.2019.00183
- Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessières et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997 Nov 20;390(6657):249-56. doi: 10.1038/36786. PMID: 9384377.
- Mormak, D A, and L E Casida. “Study of Bacillus subtilis Endospores in Soil by Use of a Modified Endospore Stain.” Applied and environmental microbiology vol. 49,6 (1985): 1356-60. doi:10.1128/AEM.49.6.1356-1360.1985
- Hiroshi Fujikawa, Diversity of the growth patterns of Bacillus subtilis colonies on agar plates, FEMS Microbiology Ecology, Volume 13, Issue 3, January 1994, Pages 159–167, https://doi.org/10.1111/j.1574-6941.1994.tb00062.x
- Lu, Zhenxiang et al. “Isolation, identification and characterization of novel Bacillus subtilis.” The Journal of veterinary medical science vol. 80,3 (2018): 427-433. doi:10.1292/jvms.16-0572
- Xiaopei Zhang, Amal Al-Dossary, Myer Hussain, Peter Setlow, Jiahe Li. Applications of Bacillus subtilis Spores in Biotechnology and Advanced Materials. Applied and Environmental Microbiology Aug 2020, 86 (17) e01096-20; DOI: 10.1128/AEM.01096-20
- Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol. 2004 Jan;50(1):1-17. doi: 10.1139/w03-076. PMID: 15052317.
- Hohmann, H.‐P., van Dijl, J.M., Krishnappa, L. and Prágai, Z. (2017). Host Organisms: Bacillus subtilis . In Industrial Biotechnology (eds C. Wittmann and J.C. Liao). https://doi.org/10.1002/9783527807796.ch7
- Outtrup, Helle & Jørgensen, Steen. (2008). The Importance of Bacillus Species in the Production of Industrial Enzymes. 10.1002/9780470696743.ch14.
