Chemical preservatives are intentional food additives incorporated into food to prevent or retard food spoilage caused by microbiological, enzymological, or chemical reactions.
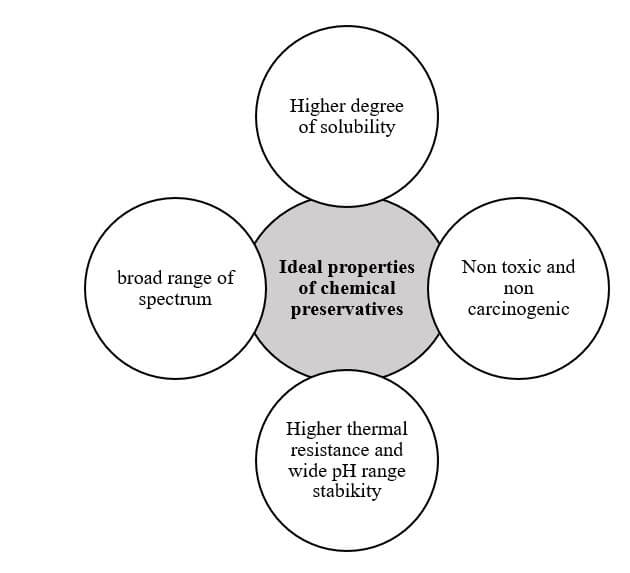
- These chemical preservatives should be nontoxic to humans or animals.
- Chemical preservatives come under the food additives generally recognized as safe (GRAS).
- Chemical preservatives can also be termed antimicrobials.
- The main purpose of using chemical preservatives is to inhibit the growth and activity of foodborne pathogens and spoilage microorganisms.
- Chemical preservatives used in food can have both bacteriostatic and bactericidal properties per the concentration used.
Interesting Science Videos
How food can get chemical preservatives?
- Intentional addition during food production, processing, or packaging
- Chemical migration from the packaging materials
- Due to a chemical reaction occurring in food
- Residues of pesticides, herbicides, and fungicides on raw food materials
- Migration of disinfectants used on utensils or equipment into foods
Role of chemical preservatives
- Interferes with the cell wall, cell membrane, enzymatic activity, nucleic acids, etc., to prevent microorganisms’ growth and activity.
- Retard, prevent or control undesirable changes in flavor, color, texture, or consistency of food and nutritive value of food.
- Control natural spoilage of food
Classification of chemical preservatives
- Class I: Traditional preservatives (natural)
- Class II: Chemical preservatives (Artificial)
Class I: Traditional Preservatives: These include preservatives like wood, smoke, sugar, honey, salt, spices, alcohol, vinegar, vegetable oil, spices, etc which are commonly used in our kitchen in past. These chemical preservatives are not restricted to use and there is no imposed limitation on their use. These naturally occurring preservatives are regarded as safe for human health.
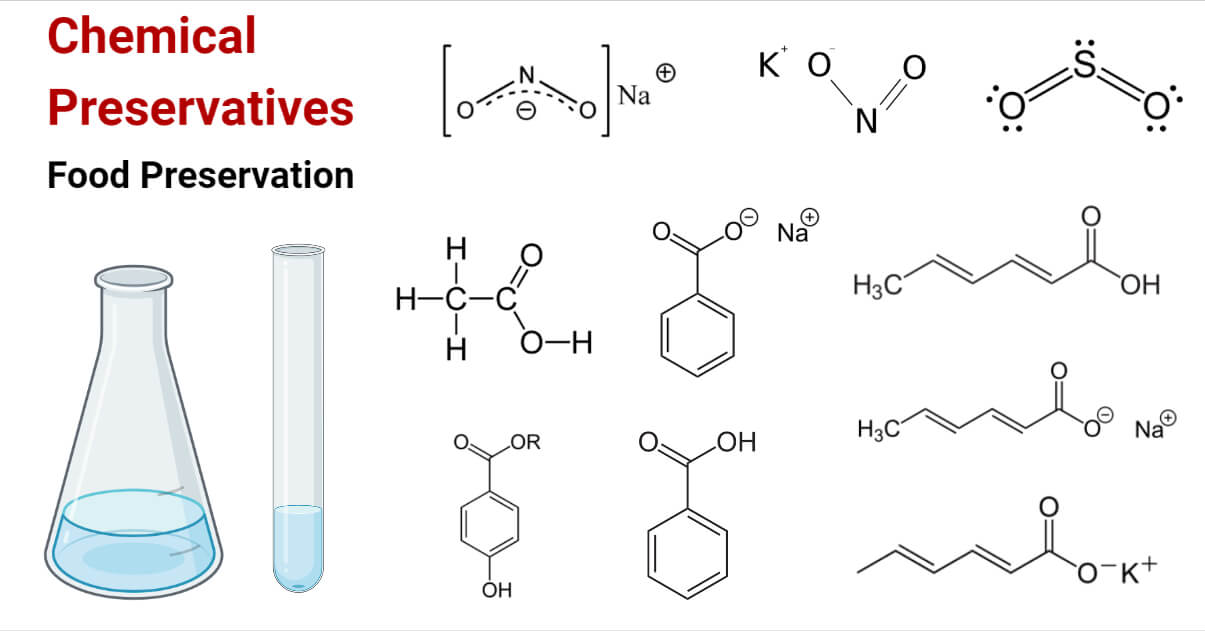
Class II: Chemical preservatives: These are synthetic chemical preservatives that are made in the laboratory. For e.g nitrites, propionates, parabens, benzoates, acetates, sorbates, sulfur dioxide, etc.
Microbial preservatives: These include antimicrobial preservatives like bacteriocins (e.g. nicin) which are produced by some strains of lactic acid bacteria and inhibit the growth of food spoilage or pathogenic bacteria. E.g nisin, produced by lactococcus lactis inhibits the growth of Clostridium tyrobutyricum, C. botulinum, and, listeria monocytogenes in cheese, other dairy products meats, fish, etc. Using bacteriocins like microbial preservatives help reduce the use of chemical preservatives like nitrates, sorbates, and benzoates which consumers consider bad.
Some food preservatives and their acceptable daily intake
Chemical preservatives with their ADI quantities (mg/kg BW). E (Europe) number refer to code for substance used as food additives. The E numbers for preservatives range from E200 to E399.
Table1: According to EU regulation, chemical food additives with their ADI quantities.
| S.N. | Chemical preservative | E number | ADI (mg/kg BW) |
| 1 | Sorbic acid | E200 | 25 |
| 2 | Sodium sorbate | E201 | 25 |
| 3 | Potassium sorbate | E202 | 25 |
| 4 | Benzoic acid | E210 | 5 |
| 5 | Sodium benzoate | E211 | 5 |
| 6 | Parabens | E214-E219 | 10 |
| 7 | Sulfur dioxide and Sulfites | E220-E228 | 0.7 |
| 8 | Potassium nitrite | E249 | 0.07 |
| 9 | Sodium nitrite | E250 | 0.1 |
| 10 | Sodium nitrate + | E251 + | 3.7 |
| 11 | Potassium nitrate | E252 | 3.7 |
| 12 | Acetic acid | E260 | |
| 13 | Propionic acid and propionates | E280- E289 | 5 |
Factors affecting the effectiveness of chemical preservatives
Chemical preservative properties
- Solubility
- Toxicity
Microbial factors
- Microbial inherent resistance to chemical preservatives
- Initial microbial load
- Growth rate and phase of microorganisms
- Stress reaction of microorganisms
- Homeostasis ability of microorganisms
- Use of additional preservative methods
Intrinsic factors of food
- pH of the food
- Water activity of food
Extrinsic factors
- Storage time and temperature
- Gas composition
- Atmosphere and relative humidity
Different chemical preservatives and their application in the food industry
| S.N | Chemical preservatives | Targeted microorganisms | Mode of action | Advantages | Disadvantages | Applications |
| 1 | Sulfur dioxide (SO2) | Yeast, mold | Increase pH and imbalance cellular metabolic process, alter the enzymatic system, | Antioxidant properties, prevent browning, preserve color, cheaper and easily available | The intense pungent odor and corrosive property makes it unuseful in canning | Beverages, fruits products, heat-sensitive foods, effective for low pH foods |
| 2 | Sorbates(Sodium sorbate and Potassium sorbate) | Yeast, Mold, Bacteria | Disturb enzyme system, inhibit many enzymes involved in TCA cycle | Beverages; juices, wines, cheese, fish meat bakery items, | ||
| 3 | Benzoic acid and benzoates | Yeast, molds | Disturb enzymatic system | Most active against yeasts and molds. Used to preserve colored fruit juices | Risk of respiratory disease | High acid foods, fruit drinks, cider, carbonated beverages, pickles, jams, salad dressings, soy sauce |
| 4 | Parabens (p-hydroxybenzoic acid) | Yeast, Mold, bacteria | Destroy complex structure of the cell and denature protein inside the cell | Soft drinks, fish products, salad dressing | ||
| 5 | Propionic acid | Mold, yeast, and a few bacteria | Disturb enzyme system | Low acid foods, processed cheese preservation | ||
| 6 | Nitrate and nitrite | Anaerobic bacteria (Clostridium botulinum), other pathogenic microbes | Inhibit metabolic enzyme | Preserve the color of red meat by forming nitrosomyoglobin | The formation of carcinogenic nitrosamines is triggering extensive research | Used in cured meats, better at low pH foods |
| 7 | Phosphates | More against gram-positive bacteria (Bacillus, clostridium) | Chelating metal ions | |||
| 8 | Sulfites | More Bacteria, less effective to yeast and mold | Target to the cytoplasmic membrane, DNA replication, protein synthesis, and enzymatic actions | Acts as antioxidants and inhibit enzymatic browning | Fruits and vegetable products, wine | |
| 9 | Sodium chloride (NaCl) | Bacteria | Osmotic shock to Plasmolysis | Better preservation if used as a pretreatment before canning, pasteurization, or drying | Weak against Staphylococcus and listeria monocytogens | Salting of meats and fish |
| 10 | Wood smoke (Traditional method) | Bacteria, fungi | The release of different phenolic compounds, ketones, aldehyde, and alcohol, which serves as an antimicrobial preservative | Easy to use | Meat, sausage, ham, bacon, fish | |
| 11 | Nisin | Clostridium botulinum and other bacteria | Cheese, cooked meat, poultry |
The working mechanism of organic acids on the bacterial cell
Organic acids like Acetic acid, benzoic acid, lactic acid, propionic acid, sorbic acid, etc., are effective as preservatives for foods with a pH of less than 5. So, they are the best for preserving acidic foods.
- At acidic pH, protonated or uncharged organic acid crosses the cell membrane and enters the cytoplasm.
- In neutral cytoplasmic pH, organic acids dissociate and release the proton that acidifies the cytoplasm.
- This cell uses ATP to pump protons out of the cell to deacidify the cytoplasm, which makes energy unavailable for their growth.
Table: Guidelines for using chemical preservatives in food by DFTQC, Nepal 2075 B.S. (2018 A.D.)
| S.N. | Food | Preservatives | PPM |
| 1 | Sausage meat containing raw meat, Cereals, spices | Sulfur dioxide | 450 |
| 2 | Undried fruits: Cherries, Strawberries, and raspberries. Other fruits | Sulfur dioxide | 2000 1000 |
| 3 | Concentrated fruit juice | Sulfur dioxide | 1500 |
| 4 | Dried fruits | Sulfur dioxide | 1500 |
| 5 | Apricots, peaches, apples, pears | Sulfur dioxide | 2000 |
| 6 | Sugar, dextrose, jaggery, refined sugar | Sulfur dioxide | 70 |
| 7 | Beer | Sulfur dioxide | 70 |
| 8 | Cider | Sulfur dioxide | 200 |
| 9 | Alcoholic wine | Sulfur dioxide | 450 |
| 10 | Dried ginger | Sulfur dioxide | 2000 |
| 11 | Squash, fruit syrups, barley water | Sulfur dioxide, or benzoic acid | 350 600 |
| 12 | Pickles | Sulfur dioxide, or benzoic acid | 250 100 |
| 13 | Jam, marmalade, fruit jelly | Sulfur dioxide, or benzoic acid | 40 200 |
| 14 | Coffee extract | Benzoic acid | 120 |
| 15 | Tomato or other juices | Benzoic acid | 750 |
| 16 | Pickled meat, bacon, canned meat | Sodium nitrite or potassium nitrite | 200 |
| 17 | Cheese or processed cheese | Sorbic acid or Sodium sorbate or potassium sorbate | 3000 |
| 18 | Paneer | Sorbic acid or Sodium sorbate or potassium sorbate | 2000 |
| 19 | Flour confectionery | Sorbic acid or Sodium sorbate or potassium sorbate | 1500 |
| 20 | Baking flour | Sodium diacetate, Propionates, Methyl propyl hydroxybenzoate | 2500 3200 500 |
Do you know?
- Nitrite and nitrate preservatives should not be used in infant foods.
- The use of titanium dioxide (E171) is fully banned as a food additive in the EU.
“Preservatives can be used to extend the expiration dates of food but unfortunately not of people.”
References
- Potter NP (1987), Food Science, CBS Pub, India
- Rahman MS (1999), Handbook of Food Preservation, Marcel Dekker, Inc, NY
- Desrosier EN (1963), The Technology of Food Preservation, AVI Publishing Company, New York
- Thomas J. Montville, Food microbiology an introduction (third edition), ASM Press, Washington, DC
- PAȘCA, C., COROIAN, A., & SOCACI, S. (2018). Risks and Benefits of Food Additives – Review. Bulletin Of University Of Agricultural Sciences And Veterinary Medicine Cluj-Napoca. Animal Science And Biotechnologies, 75(2), 71. DOI: 10.15835/buasvmcn-asb:2018.0026.
- Department of Food Technology and Quality Control (DFTQC), 2075 guideline

Where do we used citric acid?
Most the time I have using citric acid in fruits pickle is that a good thing??
Information required for sugar base energy powder