Amino acids constitute a group of neutral products clearly distinguished from other natural compounds chemically, mainly because of their ampholytic properties, and biochemically, mainly because of their role as protein constituents. An amino acid is a carboxylic acid-containing an aliphatic primary amino group in the α position to the carboxyl group and with a characteristic stereochemistry. Proteins are biosynthesized from 20 amino acids in a system involving strict genetic control. Thus, amino acids are the basic unit of proteins. More than 300 amino acids are found in nature but only 20 amino acids are standard and present in protein because they are coded by genes. Other amino acids are modified amino acids and are called non-protein amino acids. Some are residues modified after a protein has been synthesized by posttranslational modifications; others are amino acids present in living organisms but not as constituents of proteins.
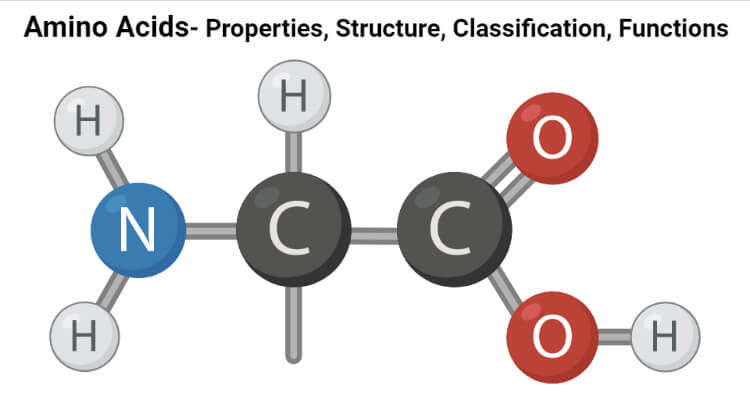
Figure: Amino Acids Back Bone.
Interesting Science Videos
Properties of Amino acids
Physical Properties
- Amino acids are colorless, crystalline solid.
- All amino acids have a high melting point greater than 200o
- Solubility: They are soluble in water, slightly soluble in alcohol, and dissolve with difficulty in methanol, ethanol, and propanol. R-group of amino acids and pH of the solvent play important role in solubility.
- On heating to high temperatures, they decompose.
- All amino acids (except glycine) are optically active.
- Peptide bond formation: Amino acids can connect with a peptide bond involving their amino and carboxylate groups. A covalent bond formed between the alpha-amino group of one amino acid and an alpha-carboxyl group of other forming -CO-NH-linkage. Peptide bonds are planar and partially ionic.
Chemical Properties
- Zwitterionic property
A zwitterion is a molecule with functional groups, of which at least one has a positive and one has a negative electrical charge. The net charge of the entire molecule is zero. Amino acids are the best-known examples of zwitterions. They contain an amine group (basic) and a carboxylic group (acidic). The -NH2 group is the stronger base, and so it picks up H+ from the -COOH group to leave a zwitterion. The (neutral) zwitterion is the usual form of amino acids that exist in the solution.
- Amphoteric property
Amino acids are amphoteric in nature that is they act as both acids and base due to the two amine and carboxylic groups present.
When 1 ml of Ninhydrin solution is added to a 1 ml protein solution and heated, the formation of a violet color indicates the presence of α-amino acids.
The xanthoproteic test is performed for the detection of aromatic amino acids (tyrosine, tryptophan, and phenylalanine) in a protein solution. The nitration of benzoid radicals present in the amino acid chain occurs due to a reaction with nitric acid, giving the solution yellow coloration.
- Reaction with Sanger’s reagent
Sanger’s reagent (1-fluoro-2, 4-dinitrobenzene) reacts with a free amino group in the peptide chain in a mild alkaline medium under cold conditions.
- Reaction with nitrous acid
Nitrous acid reacts with the amino group to liberate nitrogen and form the corresponding hydroxyl.
Structure of Amino acids
All 20 of the common amino acids are alpha-amino acids. They contain a carboxyl group, an amino group, and a side chain (R group), all attached to the α-carbon.
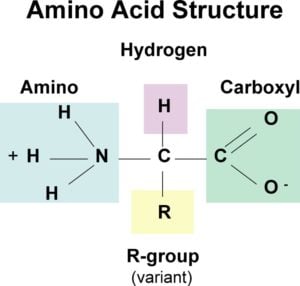
Exceptions are:
- Glycine, which does not have a side chain. Its α-carbon contains two hydrogens.
- Proline, in which the nitrogen is part of a ring.
- Thus, each amino acid has an amine group at one end and an acid group at the other, and a distinctive side chain. The backbone is the same for all amino acids while the side chain differs from one amino acid to the next.
- All of the 20 amino acids except glycine are of the L-configuration, as for all but one amino acid the α-carbon is an asymmetric carbon. Because glycine does not contain an asymmetric carbon atom, it is not optically active and, thus, is neither D nor L.
Classification of amino acids on the basis of R-group
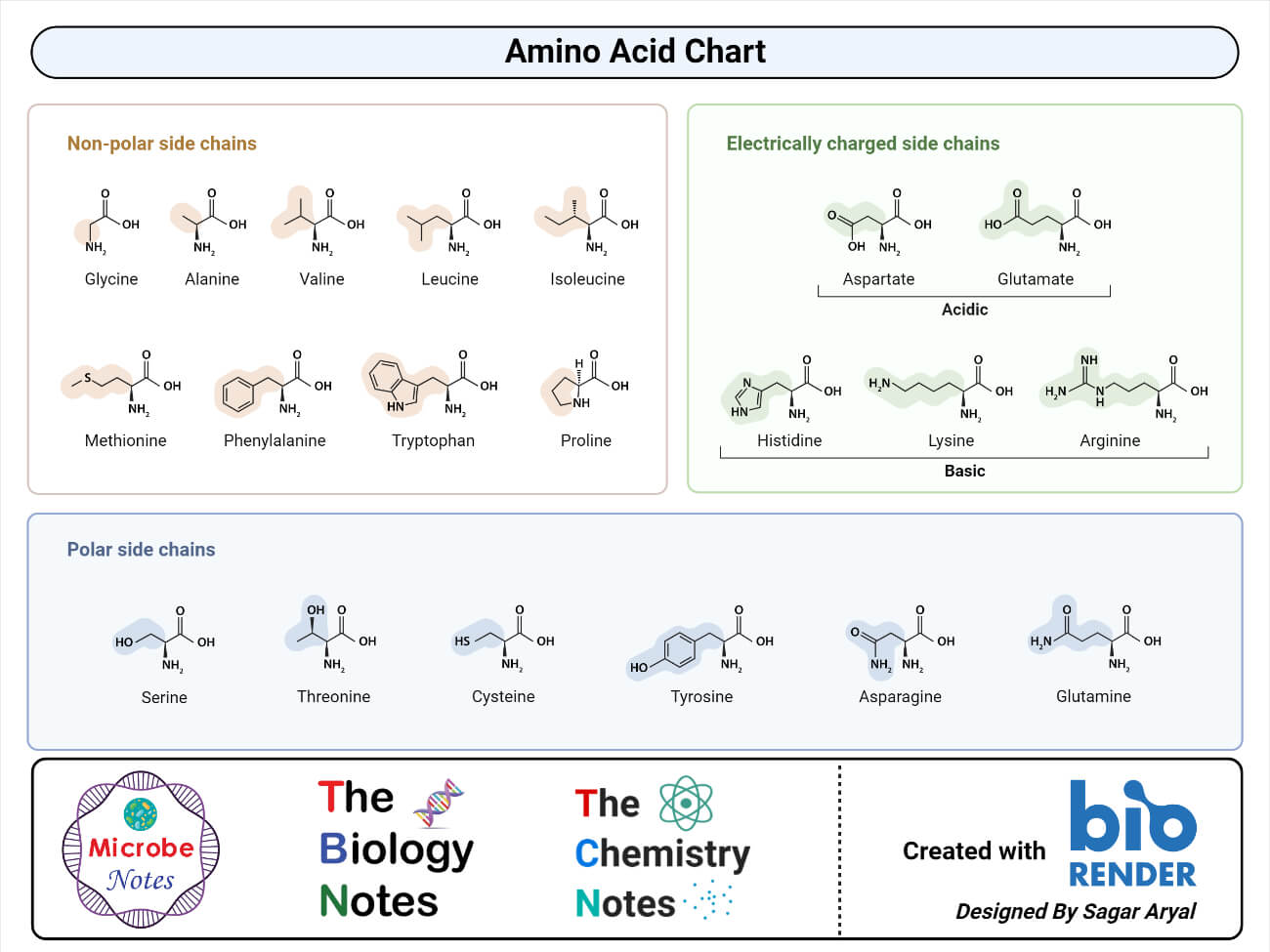
- Nonpolar, Aliphatic amino acids: The R groups in this class of amino acids are nonpolar and hydrophobic. Glycine, Alanine, Valine, leucine, Isoleucine, Methionine, Proline.
- Aromatic amino acids: Phenylalanine, tyrosine, and tryptophan, with their aromatic side chains, are relatively nonpolar (hydrophobic). All can participate in hydrophobic interactions.
- Polar, Uncharged amino acids: The R groups of these amino acids are more soluble in water, or more hydrophilic, than those of the nonpolar amino acids, because they contain functional groups that form hydrogen bonds with water. This class of amino acids includes serine, threonine, cysteine, asparagine, and glutamine.
- Acidic amino acids: Amino acids in which R-group is acidic or negatively charged. Glutamic acid and Aspartic acid
- Basic amino acids: Amino acids in which R-group is basic or positively charged. Lysine, Arginine, Histidine
Classification of amino acids on the basis of nutrition
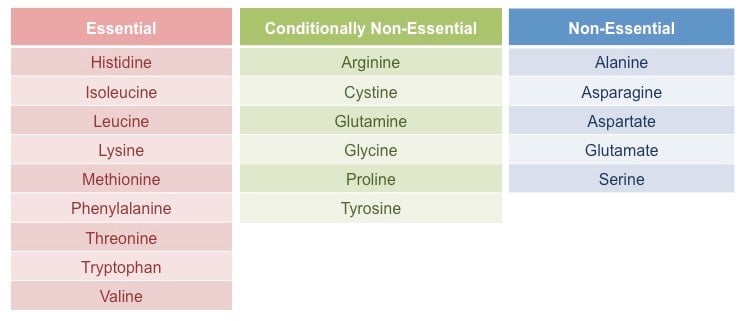
Essential amino acids (Nine)
Nine amino acids cannot be synthesized in the body and, therefore, must be present in the diet in order for protein synthesis to occur.
These essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
Non-essential amino acids (Eleven)
These amino acids can be synthesized in the body itself and hence do not necessarily need to be acquired through diet.
These non-essential amino acids are Arginine, glutamine, tyrosine, cysteine, glycine, proline, serine, ornithine, alanine, asparagine, and aspartate.
Classification of amino acids on the basis of the metabolic fate
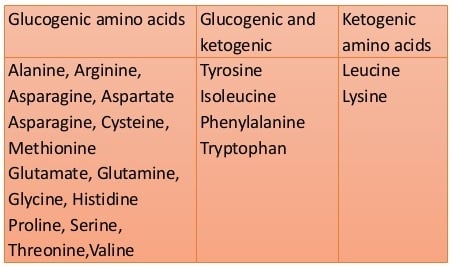
- Glucogenic amino acids: These amino acids serve as precursors of gluconeogenesis for glucose formation. Glycine, alanine, serine, aspartic acid, asparagine, glutamic acid, glutamine, proline, valine, methionine, cysteine, histidine, and arginine.
- Ketogenic amino acids: These amino acids break down to form ketone bodies. Leucine and Lysine.
- Both glucogenic and ketogenic amino acids: These amino acids break down to form precursors for both ketone bodies and glucose. Isoleucine, Phenylalanine, Tryptophan, and tyrosine.
Functions of Amino acids
- In particular, 20 very important amino acids are crucial for life as they contain peptides and proteins and are known to be the building blocks for all living things.
- The linear sequence of amino acid residues in a polypeptide chain determines the three-dimensional configuration of a protein, and the structure of a protein determines its function.
- Amino acids are imperative for sustaining the health of the human body. They largely promote the:
Production of hormones
• Structure of muscles
• Human nervous system’s healthy functioning
• The health of vital organs
• Normal cellular structure - The amino acids are used by various tissues to synthesize proteins and to produce nitrogen-containing compounds (e.g., purines, heme, creatine, epinephrine), or they are oxidized to produce energy.
- The breakdown of both dietary and tissue proteins yields nitrogen-containing substrates and carbon skeletons.
- The nitrogen-containing substrates are used in the biosynthesis of purines, pyrimidines, neurotransmitters, hormones, porphyrins, and nonessential amino acids.
- The carbon skeletons are used as a fuel source in the citric acid cycle, used for gluconeogenesis, or used in fatty acid synthesis.
References
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- http://www-plb.ucdavis.edu/courses/bis/105/lectures/AminoAcids.pdf
- http://www.aminoacidsguide.com/
- https://www.biologyexams4u.com/2012/09/amino-acids html#.W2MViTozbIV

Good work. But it would have been open to download!
Very good and excellent keep helping us
Very Clean and Informative… Thanks!
arginine and lysine needs to be switched on the structure chart
Thanks for the correction, We have updated the chart. 🙂
Thanks a lot
What a great work have here
Thanks… Though place for download could have been great
So good notes keep on helping us
Nice and clean explanation
proline is non-polar