Interesting Science Videos
What is Osmolarity?
Cells are of two parts: Intracellular and extracellular. Depending upon the tonicity of the fluid, the fluids can shift from the outside of the cell to the inside of the cell via osmosis. Osmosis is a net movement of water from a region of lower solute concentration to an area of higher solute concentration through a semipermeable membrane like a cell wall.
- Solute is the number of dissolved solids in a solution consisting of proteins, carbohydrates, ions, hormones, etc.
- The total concentration of solute is known as osmolarity.
- Low osmolarity in a solution has low solute particles per liter solution, while high osmolarity has high solute particles per liter solution.
- Osmotic pressure is the hydrostatic pressure between solutions separated by a semipermeable membrane.
Hypotonic Solution
The term hypotonic has two parts: hypo means “less/under/beneath,” and tonic means “stretching or concentration of a solution.”
A solution with a lower solute concentration or lower osmotic pressure across a semipermeable membrane is called a hypotonic solution. A solution is known as hypotonic only when compared to the solute concentration of another solution.
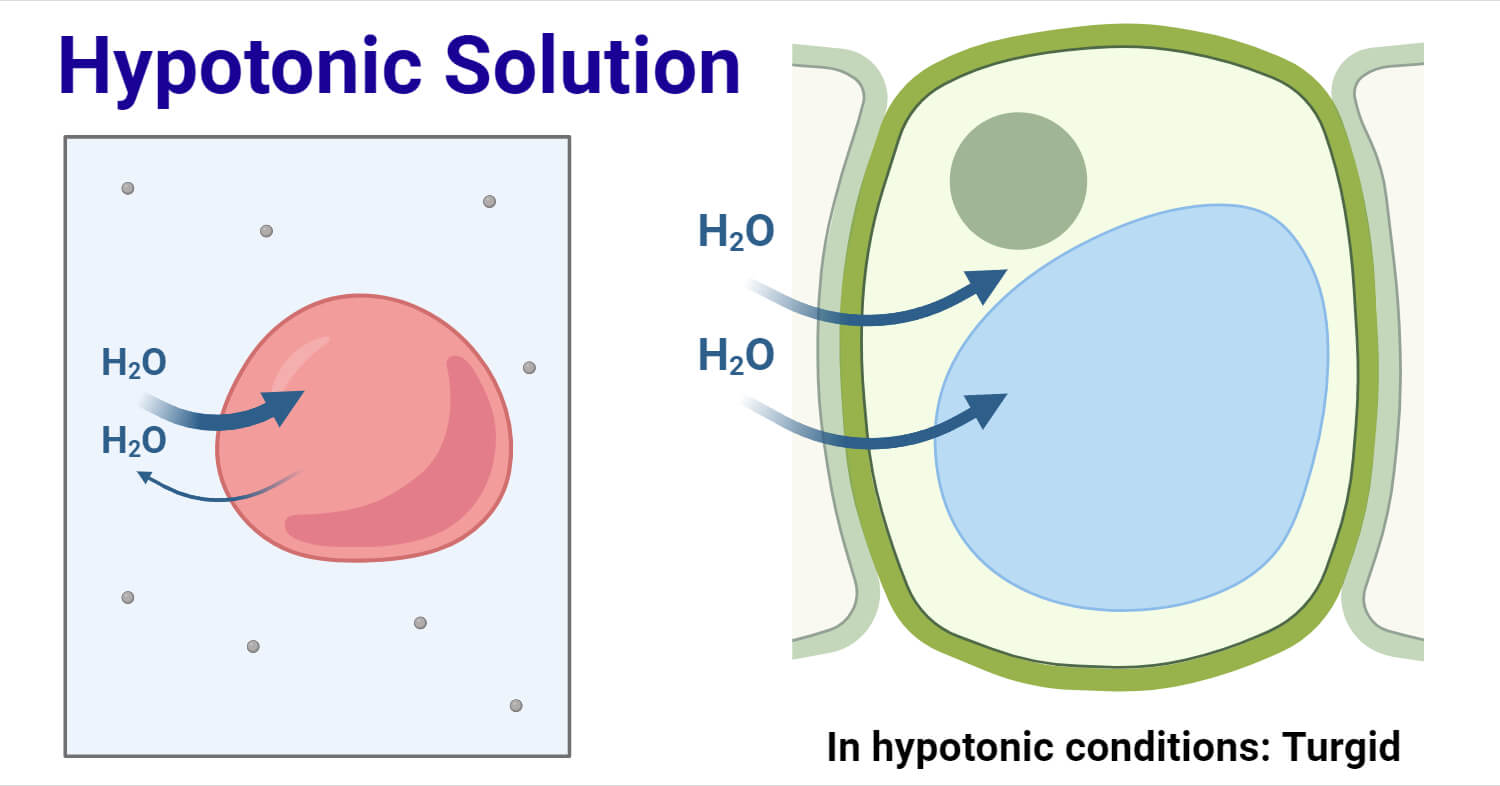
Scientists can determine which direction the water gradient and solute gradients will form with the help of measuring or knowing the osmolarity (concentration of a solution in the number of solutes per liter) of different solutions. Water enters the cell because of the difference in concentration between the compartments.
- When a cell is kept in a hypotonic solution, a net flow of water will enter the cell (the cell absorbs the hypotonic solution) through a cell wall.
- The extracellular fluid has lower osmolarity and a higher water concentration in the solution than the cell does. It causes the water to follow its concentration gradient.
- In this condition, the continuous diffusion of the water molecule into the cell leads to cell swelling.
- Both plant and animal cells are eukaryotic cells. The solution is hypotonic to the cell if the concentration of solutes outside the cell is lower than the concentration inside the cell, and the solutes are not able to pass through the membrane.
- Animal cells cannot regulate their intake, whereas plant cells can regulate. In contrast to animal cells, plant cells have a rigid cell wall, which prevents them from bursting.
- In contrast to the cell, the environment may be a hypotonic solution. The environment’s water tends to diffuse into the cell. The cell may lyse (split open) if the hypotonic solution in the environment is too strong.
- Cells have many mechanisms to regulate this flow of water.
Hypotonic Solutions
- 0.45% Saline (1/2 NS)
- 0.225% Saline (1/4 NS)
- 0.33% saline (1/3 NS)
Examples of Hypotonic solution
1. Body Fluids
Using bodily fluids as a baseline, some examples of hypotonic solutions are:
- Pure water
- Plain, unsweetened tea
- Sports drinks that are low in sugar and salt
2. Animal Systems
- Animal cells lack cell walls, as they are more susceptible to rupture in strongly hypotonic environments.
- Animals use their skin to provide a barrier between their internal organs and the external environment. Then, a network of membranes and proteins can control the fluid inside their body cavity.
- Animals have evolved osmoregulatory mechanisms to combat this. It allows them to keep solute concentrations within ideal limits and keep them plump and healthy without destroying them.
Marine animals
- Numerous marine animals, such as sharks and sea turtles, have salt glands that allow them to eliminate extra salt from their bodies.
- To get the water into their bodies, animals must drink salt water/seawater.
- However, the salts must be concentrated and eliminated from the body to keep their internal environment more hypotonic than seawater.
- Because of this adaption, they can absorb water from their surrounding and keep their cells hydrated.
Protists
- Amoeba and paramecia are examples of single-celled organisms that live in hypotonic environments.
- They lack robust structural components like cell walls or cytoskeletons.
- Contractile vacuoles are specialized structures that regulate the continuous inflow of water into cells by periodically releasing excess water to prevent the rupture of cells.
Freshwater fishes
- Freshwater fishes such as catfish and trout thrive in hypotonic environments.
- These fishes have developed systems to continuously expel excess water from their bodies, maintaining cellular homeostasis.
Humans
- The human renal system plays a crucial role in osmoregulation, particularly the kidneys.
- Osmoregulation is the process of maintaining the solute concentration in an organism.
- It also maintains solutes in the body.
- Kidneys regulate blood osmotic pressure, maintaining water balance and essential mineral ions through intricate filtration and reabsorption processes.
3. Plant and Fungi
- In a hypotonic solution, plant cells respond similarly to animal cells. But the effects might not be as severe.
- Plants and fungi have developed mechanisms to live and survive in hypotonic environments.
- They actively regulate the solute concentration around their cells to ensure that the extracellular environment is always a hypotonic solution compared to cells.
- This regulation results in turgid cells. Due to the influx of water, it exerts pressure against their cell walls.
- This turgidity helps to maintain the upright posture of plants and is crucial for non-woody plants that lack the structural support of lignified tissues.
- Plants have rigid cell walls made of cellulose. It covers the plasma membrane. It also contains a vacuole that stores excess water.
- It plays a crucial role in maintaining this balance. As a result, the cells find it more difficult to lyse, but the extra pressure leads the cell’s sides to protrude.
- Similarly, fungi, with their chitinous cell walls, control water inflow to maintain the structural integrity of their cells.
- Bryophytes, like mosses, are devoid of vascular tissues. To facilitate the upward transport of water through capillary action, they mainly depend on the external water supply and the hypotonic nature of their surroundings.
References
- Biology Dictionary. (2019, October 5). Hypotonic solution. Retrieved from https://biologydictionary.net/hypotonic-solution/
- Tonicity: hypertonic, isotonic & hypotonic solutions (article) | Khan Academy. (n.d.). Retrieved from https://www.khanacademy.org/science/ap-biology/cell-structure-and-function/mechanisms-of-transport-tonicity-and-osmoregulation/a/osmosis
- Biology Dictionary. (2019, October 5). Isotonic vs. Hypotonic vs. Hypertonic Solution. Retrieved from https://biologydictionary.net/isotonic-vs-hypotonic-vs-hypertonic-solution/
- McKinsey, M. (2023, January 12). Hypotonic Solution — Definition & Examples (Cells). Retrieved from https://tutors.com/lesson/hypotonic-solution-definition-examples
- Admin. (2022, July 8). What is a Hypotonic Solution? – BYJU’S. Retrieved from https://byjus.com/neet/hypotonic-solution/
- Mazurek, D. (2023). Hypotonic vs. Hypertonic vs. Isotonic: Learn The Difference. In Dictionary.com. Retrieved from https://www.dictionary.com/e/hypotonic-vs-hypertonic-vs-isotonic/
- Isotonic, Hypotonic & Hypertonic IV Fluid Solution NCLEX Review Notes. (2023, July 18). Retrieved from https://www.registerednursern.com/isotonic-hypotonic-hypertonic-iv-fluid-solution-overview-for-nursing-students-with-quiz/
- Vedantu. (n.d.). Hypotonic solution. Retrieved from https://www.vedantu.com/biology/hypotonic-solution
- Biology Dictionary. (2018, May 6). What happens to a cell in a hypotonic solution. Retrieved from https://biologydictionary.net/what-happens-to-a-cell-in-a-hypotonic-solution/
- Hypotonic Solution – definition and examples, types. (2021, February 1). Retrieved from https://www.toppr.com/guides/chemistry/solutions/hypotonic-solution-definition-and-examples/
