Osmosis is a biophysical process occurring commonly in biological systems where solvent molecules move across a semi-permeable membrane towards a region of high solute concentration.
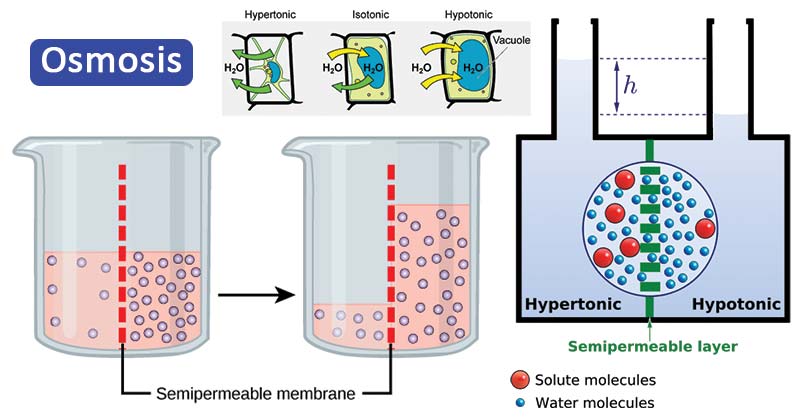
Figure: Osmosis. Image Source: Open Stax (Rice University) and Biology Online.
- It is a type of passive transport and is directed towards the direction that tends to equalize the solute concentration across a semi-permeable membrane.
- It is a common process taking place in most of the biological membrane in the organisms.
- In a biological system, the solvent mostly is water; however, osmosis can also take place in other liquids and even gases.
- As it is a means of passive transport, it doesn’t require any energy.
Interesting Science Videos
Mechanisms of osmosis (How does osmosis work?)
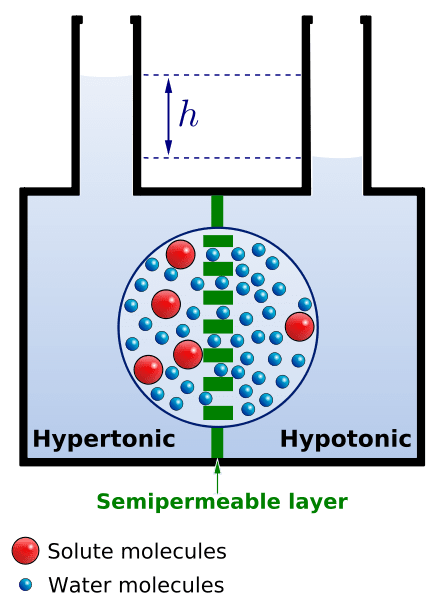
Figure: A schematic diagram showing how osmosis works. Image Source: Biology Online.
- In order to describe the process and mechanism of osmosis, we take two solutions separated by a semi-permeable membrane.
- One of the solutions is pure water while the other one is a solution of some solute and water.
- According to the definition of osmosis, in such a case, pure water moves across the membrane towards the solute solution.
- Several descriptions have been made to understand the driving force of osmosis. One such theorem explains that the movement of water across the membrane results due to the concentration gradient of water in the two solutions.
- This theorem, however, doesn’t explain the process of reverse osmosis, which occurs against the concentration gradient with the movement of solvent towards the solution of lower concentration.
- Another theorem put forth by several other scientists is the fact that the solute molecules in the solution attract the solvent molecules across the membrane. This theorem too doesn’t hold true as the size of the solute molecules does not influence the movement of a solvent across the membrane.
- Therefore, the process of osmosis has been explained with the concept of chemical potential.
- The chemical potential of pure water in one solution differs from the chemical potential of water in the solution with solute molecules.
- The interaction between the solute and water molecules reduces the pressure exerted by the water molecules in the solute solution. As a result, the water molecules in the pure water exert more pressure towards the solution with lower solvent concentration.
- This pressure results in the forcing of water across the membrane. This process continues until the pressure on both sides becomes equal leading to equilibrium.
Factors affecting osmosis
Osmosis is a result of various factors, and thus the rate of osmosis is influenced by a number of such factors:
Temperature
- The rate of osmosis increase with the increase in temperature of the system.
- This occurs because, with the increase in temperature, the energy of the molecules also increases.
- As the molecules become more energetic, their movement also increases, and thus the process of osmosis is escalated.
Concentration gradient
- As the concentration of solute molecules is essential in the driving force of osmosis, any changes in the concentration directly affect the rate of osmosis.
- Osmosis shoots up in a condition where the difference in concentration of solute across the membrane is higher.
- As the number of solute molecules is more in one solution than the other, the pressure exerted by the solvent molecules decreases, thus accelerating the process of osmosis.
- Once equilibrium is maintained across the membrane, the process of osmosis ceases.
Water potential/ Solvent potential
- The water potential across a semi-permeable membrane also influences the rate of osmosis.
- As the water potential of a solution is more, the water molecules can move across the membrane as the pressure exerted by the particles is increased.
- Eventually, the water potential on either side becomes equal, creating equilibrium.
- Once equilibrium is reached, water continues to flow across the membrane, but it flows both ways in equal amounts, thus stabilizing the solutions.
Surface area and thickness of the membrane
- With the increase in surface area, more space will be available to the molecules for their movement which in turn will increase the rate of osmosis.
- Similarly, if the surface area is reduced, less space will be there for the molecules to move, which will restrict their movement.
- The rate of osmosis is also reduced as the thickness of the membrane increases.
Pressure
- The pressure is an essential factor influencing the process of osmosis as it might even change the direction of osmosis.
- If the pressure is applied in excess to the pressure applied by the solvent molecules, the direction of osmosis might change, and the solvent molecules begin to move towards the region with more solvent concentration.
- However, if pressure less than that exerted by the solvent molecules is applied, it doesn’t change the direction but does reduce the rate of osmosis.
- The pressure applied in the same direction of the concentration gradient also increases the rate of osmosis.
Variation/ Types of osmosis
There are some variations or types of osmosis on the basis of the direction of the movement of solvent molecules.
Reverse osmosis and Forward osmosis
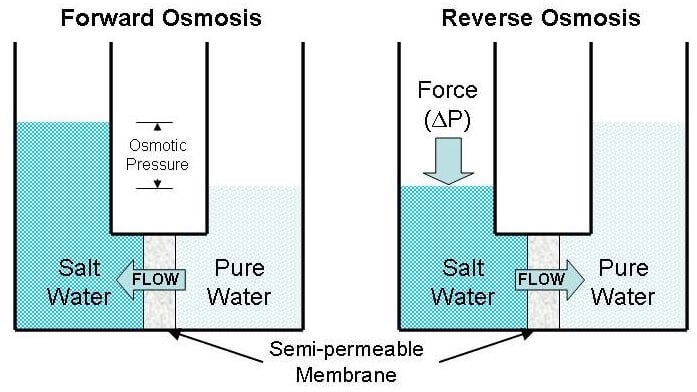
Image Source: AIChE.
- Reverse osmosis is a method of separation that is used to force a solvent through a semi-permeable membrane resulting in solute molecules on one side and solvent molecules on the other side.
- Reverse osmosis is different than the forward osmosis in that reverse osmosis utilized hydraulic pressure to force the solvent against the osmotic pressure.
- Forward osmosis is another variation of osmosis where the osmotic pressure gradient is used to induce the flow of water from the sample solution to separate the solutes.
- Forward osmosis uses a draw solution with a higher concentration of solute, which extracts the solvent molecules from the sample solution; thus, resulting in the separation of solute and solvent in the sample solution.
Endosmosis and exosmosis
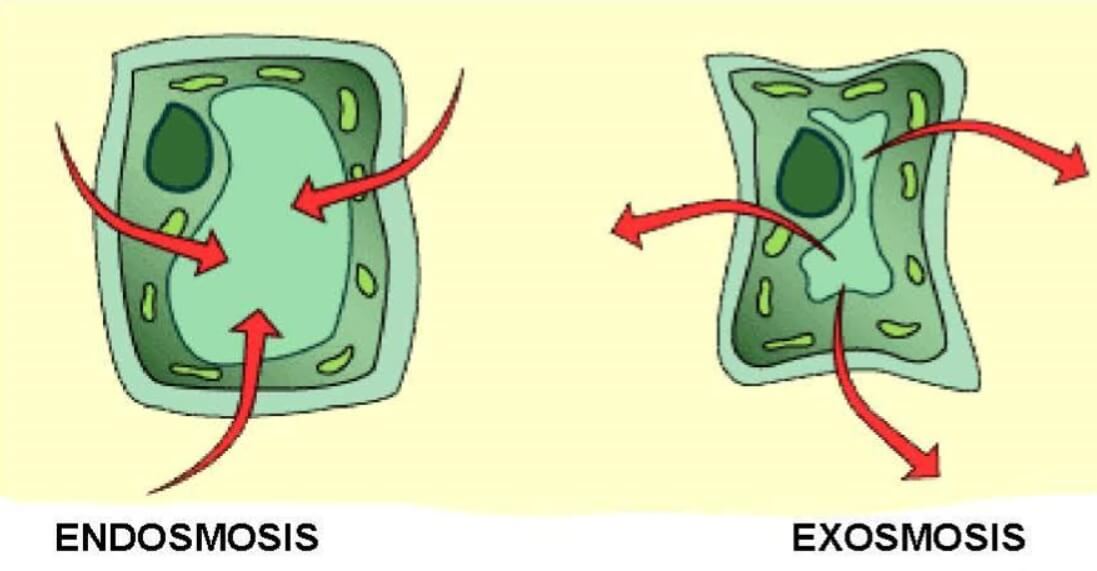
Image Source: Toppr.
- Endosmosis is the movement of water into the cell which occurs when a cell is placed in a solution having a higher concentration of water than the cell.
- Exosmosis is the movement of water out of the cell which occurs when a cell is placed in a solution having a higher concentration of solute than the cell.
- Cells swell up in size after endosmosis while the cells shrink after exosmosis.
Osmotic solutions (Tonicity)
- Tonicity is the ability of extracellular solutions to induce the movement of water in and out of a cell by the process of osmosis.
- The tonicity of a solution is determined the concentration of solute and solvent molecules in the solution.
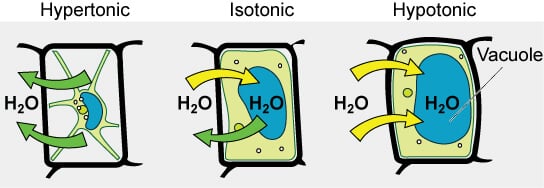
Image Source: Open Stax (Rice University)
On the basis of the tonicity of solutions, they are termed as hypotonic, hypertonic, or isotonic solutions.
Hypotonic solution
- If an extracellular solution has less concentration of solute than that inside the cell, the solution is termed a hypotonic solution.
- When a cell is placed in a hypotonic solution, the movement of water occurs into the cell resulting in endosmosis.
- The cell is such a condition will swell up and might even burst.
Hypertonic solution
- If an extracellular solution has more concentration of solute than that inside the cell, the solution is termed a hypertonic solution.
- When a cell is placed in a hypertonic solution, the movement of water occurs out of the cell resulting in exosmosis.
- The cell shrinks down, losing the ability to divide, and even function.
Isotonic solution
- When an extracellular solution has the same concentration of solute as that inside the cell, the solution is termed an isotonic solution.
- When a cell is placed in an isotonic solution, no movement of water occurs across the cell membrane.
- In this case, the size of the cell is not influenced as no movement of water takes place.
Osmotic Pressure
- Osmotic pressure is the pressure applied by a hypotonic pressure that results in the movement of solvent molecules across the semi-permeable membrane.
- It is the minimum pressure that should be applied to the solution to prevent the inwards flow of pure solvent across the semi-permeable membrane.
- Osmotic pressure is the driving force of osmosis and the rate of osmosis increases as the osmotic pressure increases.
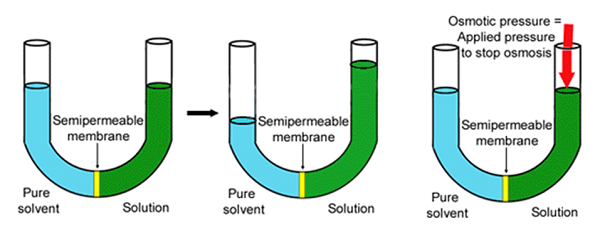
Image Source: Pharmaceuticalmicrobiologi.
The osmotic pressure of a solution can be calculated as follow:
∏= MRT
Where ∏ is the osmotic pressure
M is the molar concentration of the solute
R is the gas constant
And T is the temperature of the system.
Significance of osmosis
Osmosis has the following significances in the chemical and biological systems:
- Osmosis is responsible for the transportation of nutrients into the cell and waste materials out of the cell.
- Osmosis influences the transport of water from the soil into the roots of plants which is then conducted to different parts of the cell via the xylem tissue.
- The internal environment of the cell in living organisms is stabilized by the balance between water and the intracellular fluid levels.
- Osmosis is also responsible for maintaining the turgidity of the cell.
- Osmosis in plants prevents the cells from drying out as a result of water loss by transpiration.
- The cell to cell diffusion of water and other cellular fluids is also maintained by osmosis.
- The movement of plants and plant parts is regulated by the turgidity of the cell, which in turn is balanced by osmosis.
- Osmosis also prevents the desiccation of fruits and sporangia among other plant structures.
- An increased osmotic pressure supports the plants in the desert areas against drought and other such injuries.
- Reverse osmosis and forwards osmosis are methods of separation used in the purification of drinking water, desalination, wastewater purification, the concentration of liquid foods like juices, production of maple syrups, low alcohol beer and hydrogen peroxide.
Examples of osmosis
In the animal cells
- Osmosis influences the shape and size of animal cells as there is no cell wall in animal cells.
- The red blood cells in humans are highly influenced by the osmotic pressure of blood. If the blood is too dilute, the RBCs shrink in size while they swell up and even burst if the blood is concentrated.
- Thus, in animals, the concentration of body fluids; blood plasma and tissue fluid, should be kept within strict limits.
- Another example of osmosis in animals is the shrinking of slugs on exposure to salt.
- The skin of slugs is a semi-permeable membrane that on exposure to salt, draws out water from the cells resulting in the shrinking of the cell and, in turn, the animal.
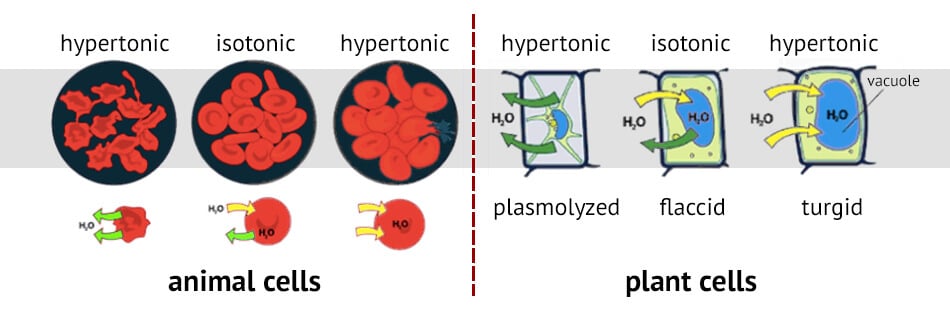
Figure: Effects of osmosis on cells in different tonic environments. Image Source: Philpot Education.
In the plant cells
- The root system in plants takes up water from the soil via osmosis.
- The cells in the root of the plants have a semi-permeable membrane that allows the water in the soil to infiltrate into the roots influencing the guard cells.
- Another classic example of osmosis in plants is the swelling up and shrinking of potato cells when slices of potato are dipped in a hypotonic solution and hypertonic solutions respectively.
Osmosis Video Animation
Osmosis vs. Diffusion
| Basis for comparison | Diffusion | Osmosis |
| Definition | Simple diffusion is a type of passive transport in which the movement of solute occurs when its electrochemical potentials on the two sides of a permeable barrier are different. | Osmosis is a type of passive transport occurring commonly in biological systems where solvent molecules move across a semi-permeable membrane towards a region of high solute concentration. |
| Nature of the membrane | Diffusion occurs through any permeable membrane. | Osmosis requires a semi-permeable membrane. |
| Nature of the process | Diffusion is a passive process. | Osmosis is also a passive process. |
| Medium | Diffusion can take place in all mediums (solid, liquid, and gas). | Osmosis only occurs in a liquid medium. |
| Type of diffusing molecules | The moving molecules can be either of solid, liquid or gases. | The moving molecules in osmosis are always liquid molecules. |
| Rate of the process | Diffusion is faster than osmosis. | Osmosis is slower than diffusion. |
| Driving force | The driving force of diffusion is the concentration gradient. | The driving force of osmosis is osmotic pressure. |
| Direction of movement | Diffusion takes place in all directions. | Osmosis takes place in one direction. |
| Control of the process | Diffusion cannot be stopped or reversed by any pressure. | Osmosis can be stopped and even reversed by applying pressure equal to or more than the osmotic pressure. |
| Types of solution | Diffusion can take place between similar or dissimilar solutions | Osmosis takes place only between two similar solutions. |
References
- Friedman, M. (2008). Principles and models of biological transport. Springer.
- https://en.wikipedia.org/wiki/Osmosis#Mechanism
- https://byjus.com/biology/osmosis/
- https://biodifferences.com/difference-between-osmosis-and-diffusion.html
- https://byjus.com/biology/difference-between-diffusion-and-osmosis/

