IMViC test is a series of four different biochemical tests used in identifying and differentiating bacteria, especially the members of Enterobacteriaceae. Though it can be (and is) used for the identification of any type of bacteria, it is mainly used for identifying Gram-negative bacteria. It is the key to identifying and differentiating members of the Enterobacteriaceae family.
IMViC is an acronym for four different biochemical tests; each letter except “I” represents an individual test making this series of biochemical tests. IMViC series contains the following biochemical tests:
- “I” = Indole Test
- “M” = Methyl Red (MR) Test
- “V” = Voges – Proskauer (VP) Test
- “C” = Citrate Utilization Test (simply Citrate Test)
The letter “i” after ‘V’ is only for the rhyming purpose, it does not indicate any test.
On making a minor modification like the use of Sulfide-Indole-Motility (SIM) Agar medium, instead of 4, we can test 6 biochemical properties. The use of SIM medium gives information on the ability to test bacteria’s ‘motility’ and ‘sulfide production’ (H2S production) ability. Hence, it is a widely accepted series of biochemical tests.
IMViC is the most widely used primary biochemical test series. Though considered key to selectively differentiating Enterobacterales, it is also used for characterization and identification of several Gram-positive bacteria. It is routinely used in clinical laboratories for teaching and research purposes. All the tests in the series are easy to perform and give results within 24 – 48 hours. Hence, used for the primary screening purpose.
Interesting Science Videos
Objectives of IMViC Test
- To study some biochemical properties – indole production, acid production, acetylmethylcarbinol (acetoin) production, and citrate utilization – of isolated unknown bacteria in order to characterize and identify them.
- To selectively differentiate and identify members of the Enterobacteriaceae family.
Principle of IMViC Test
The IMViC test is based on the variations in the metabolic requirements and properties of different genera and species of bacteria. The ‘indole test’ and ‘citrate utilization test’ in the series detect the ability of bacteria to produce specific enzymes and utilize specific nutrients. On the other hand, the ‘MR test’ and ‘VP test’ in the series detect the final metabolic products produced by the bacteria utilizing specific nutrients. For this purpose, the test bacteria is cultured in specific culture media; different media for the different tests (expect MR and VP tests which need the same culture media; MR-VP media).
Requirements for IMViC Test
The IMViC test is a series of different biochemical tests requiring different culture media and reagents. Traditionally, broths were only used but now different solid media are widely recommended for ease and ability to test multiple properties.
Requirements for Indole Test:
Culture media:
- Tryptophan broth was traditionally used.
- Recently, Sulfide – Indole – Motility (SIM) medium is widely recommended (because it gives the result of H2S production and motility also).
- Motility – Indole – Urea (MIU) medium is also preferred (because it gives the result of urease production and motility also).
Reagents:
- Kovac’s Indole Reagent (a solution of 4- (dimethylamino)benzaldehyde and hydrochloric acid in n-butanol or amyl alcohol) is mostly used. It is preferred for aerobic organisms.
- Ehrlich’s Reagent (a solution of p-dimethylaminobenzaldehydre and hydrochloric acid in ethyl alcohol) is preferred for anaerobic and weak indole producing organisms.
- 5% p-dimethylaminobenzaldehyde or 1% pdimethylaminocinnamaldehyde in 10% (v/v) concentrated HCl for the spot indole test.
Requirements for MR Test:
Culture media:
- MR-VP broth
Reagents:
- Methyl red indicator
Requirements for VP Test:
Culture media:
- MR-VP broth
Reagents:
- Barritt’s A solution or VP reagent I (5% – α-naphthol solution)
- Barritt’s B solution or VP reagent II (40% KOH solution)
Requirements for Citrate Utilization Test:
Culture media:
- Simmon’s Citrate Agar
Reagent:
- Bromothymol blue indicator (it is already incorporated in the Simmon’s citrate medium)
- The simplified IMViC agar plate containing modified media containing all four IMVic test media is also prepared, but it is not applied widely.
What is Indole Test?
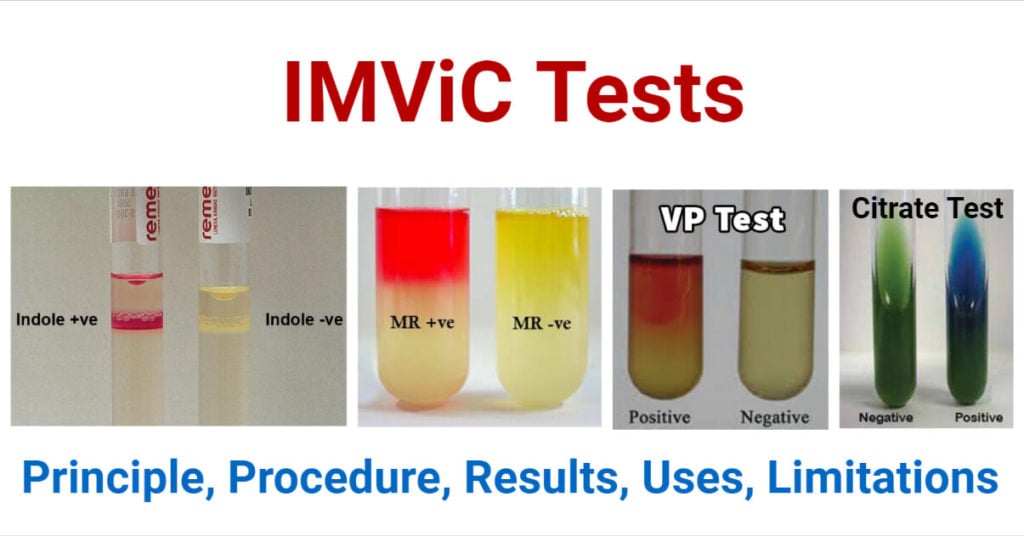
The indole test is a biochemical test in the IMViC test series which detects the ability of organisms (bacteria) to produce indole as a metabolic product utilizing tryptophan. It is indicated by the letter “I” of the IMViC.
Principle of Indole Test
Some bacteria can produce an enzyme called ‘tryptophanase’ which helps them to metabolize the amino acid ‘tryptophan’ into ‘indole, pyruvic acid, and ammonia’.

When the indole reagent is added to a medium with a bacterial culture that has produced indole, the indole combines with the aldehyde present in the reagent to give a distinctive color.
If benzaldehyde is present in the reagent, a pink to a violet-red quinoidal compound is formed, hence; a pink to red color ring is formed.
If cinnamaldehyde is present in the reagent, a blue to the green color compound is formed, hence; a green to blue color ring is formed.
(Kovac’s indole reagent uses amyl alcohol and benzaldehyde. The amyl alcohol is water-insoluble and forms an oily layer, thus giving a cherry-red or pink – red ring on the top.)”
Test bacteria are cultured in the medium containing tryptophan for 24 – 48 hours and an indole reagent is added following the incubation to read the result. A positive result is indicated by the formation of a pink to violet-red or green to blue color ring according to the type of reagent used. A negative color is indicated by the formation of a lack of color change or a slight yellowish color ring at the top.
Indole Positive Bacteria: Escherichia coli, Klebsiella oxytoca, V. cholerae, Proteus vulgaris, Porphyromonas asaccharolytica, Vibrio spp., Flavobacterium spp., Providencia spp., Enterococcus faecalis, Haemophilus influenzae, Morganella morganii, Aeromonas spp., Citrobacter koseri,
Indole Negative Bacteria: Klebsiella pneumoniae, Proteus mirabilis, Salmonella spp., Shigella spp., Citrobacter freundii, Pseudomonas aeruginosa, Bacteroides fragilis, Staphylococcus aureus,
Read More: Indole Test- Principle, Media, Procedure, Types, Results, Uses
What is Methyl Red (MR) Test?
Methyl Red (MR) Test is a biochemical test that detects the ability of organisms (bacteria) to produce stable mixed acids as metabolic end products of glucose metabolism. It is indicated by the letter “M” of the IMViC.
Principle of MR Test
Some species of bacteria use the mixed acid fermentation pathway as their glucose metabolism process. Following this metabolic pathway, they convert pyruvate into stable mixed acids.

When such acid fermenters bacteria are grown in a medium containing glucose (or carbohydrate), they will release the acids, hence; decreasing the pH of the medium to 4.4 or lower. When methyl red indicator is added in a medium containing such acid fermenters, it will turn the medium red.”
Following the 24-hour incubation on the MR-VP broth, a methyl red indicator is added to the broth. A positive result is indicated by the development of red color while a negative result is indicated by the development of yellowish color.

MR Positive Bacteria: Escherichia coli, Salmonella spp., Shigella spp., Citrobacter spp., Proteus spp., Yersinia spp., Edwardsiella spp., Staphylococcus aureus,
MR Negative Bacteria: Klebsiella pneumoniae, Enterobacter spp., Hafnia spp., Serratia marcescens.
Read More: Methyl Red (MR) Test- Principle, Procedure, Results, Uses
What is Voges-Proskauer (VP) Test?
Voges-Proskauer (VP) Test is a biochemical test in the IMViC test series which detects the ability of organisms (bacteria) to metabolize the pyruvate into a neutral intermediate product called ‘acetylmethylcarbinol’ or ‘acetoin’. It is indicated by the letter “V” of the IMViC.
Principle of VP Test
Pyruvate can be metabolized into a neutral intermediate product called ‘acetyl methyl carbinol’, commonly called the ‘acetoin’ during the butanediol pathway of 2,3-butanediol production.
If acetoin is present in the media, it is oxidized readily to diacetyl in presence of air and KOH. Thus produced diacetyl, in the presence of ∝ – naphthol, will react with the guanidine component of peptone forming a pink to a red colored product.
Following the 48-hour aerobic incubation on MR-VP broth, VP reagents I and II are added and the color change is observed within 30 minutes. A positive result is indicated by the development of pink – red color at the top of the broth immediately or within 30 minutes but not more than 1 hour. No change in color represents a negative VP test.

VP Positive Bacteria: Klebsiella spp., Enterobacter spp., Viridans Streptococci (except S. mitis, and S. vestibularis), Proteus mirabilis, Hafnia spp., Serratia spp., Staphylococcus aureus,
VP Negative Bacteria: Escherichia spp., Proteus vulgaris, Citrobacter freundii,
Read More: Voges Proskauer (VP) Test- Principle, Procedure, Results, Uses
What is Citrate Utilization Test?
Citrate Utilization Test is a biochemical test in the IMViC test series which detects the ability of organisms (bacteria) to utilize citrate as a sole source of energy. It is indicated by the letter “C” of the IMViC.
Principle of Citrate Utilization Test
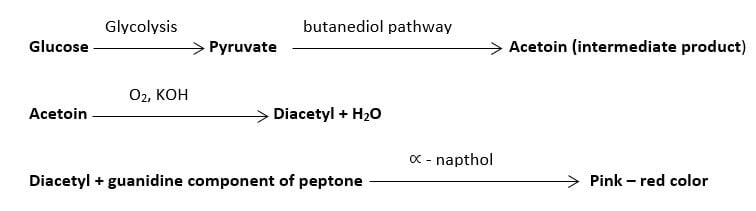
Some bacteria can utilize ‘citrate’ as their sole source of carbon. Such bacteria produce citrase enzymes which will break the citrate into oxaloacetic acid and acetic acid. The oxaloacetic acid will then be decarboxylated to produce pyruvate and CO2.

Released CO2 will combine with H2O and excess sodium from sodium citrate to produce alkaline ‘sodium carbonate’. The sodium carbonate will increase the pH of the medium.
CO2 + H2O + excess sodium from sodium citrate → Na2CO3 (alkaline)
Additionally, the released CO2 will trigger the metabolism of ammonium salts. Utilization of the ammonium salts as a source of nitrogen will cause the production of ammonia (or ammonium hydroxide).
Ammonium salt → Ammonium hydroxide (alkaline)
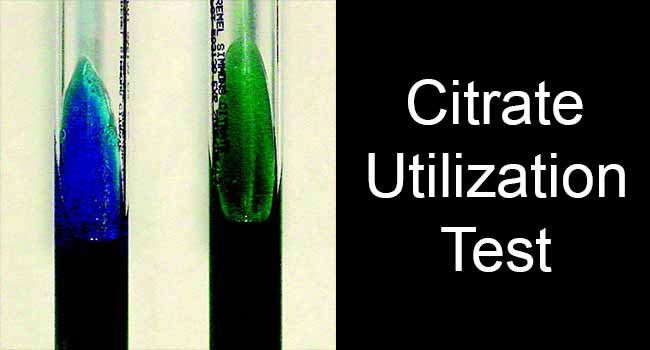
The combined effect of ammonium hydroxide and sodium carbonate will increase the pH of the media above 7.6. This increase in pH will turn the pH indicator bromothymol blue in the medium from deep forest green (at neutral pH) to Prussian blue.

Following the incubation of 24 – 48 hours (up to 4 days for some), bacterial growth and color change in the slant portion is observed. A positive result is indicated by growth and change in color of slant from green to intense blue. A negative result is indicated by no change in the color of the slant.
Citrate Positive Bacteria: Klebsiella spp., Citrobacter spp., Serratia marcescens, Proteus mirabilis, Enterobacter spp., Salmonella spp. (except Salmonella Typhi and Paratyphi A), Edwardsiella spp., Providencia spp.,
Citrate Variable Bacteria: Proteus vulgaris, V. cholera, V. parahaemolyticus.
Citrate Negative Bacteria: Escherichia coli, Shigella spp., Salmonella Typhi and Paratyphi A, Yersinia spp., Morganella morganii, Staphylococcus aureus, etc.
Read More: Citrate Utilization Test- Principle, Procedure, Results, Uses
IMViC test result chart of some common bacteria
| Name of Bacteria | Indole Test | MR Test | VP Test | Citrate Test |
| E. coli | + ve | + ve | – ve | – ve |
| Klebsiella pneumoniae | – ve | – ve | + ve | + ve |
| Klebsiella oxytoca | + ve | – ve | + ve | + ve |
| Pseudomonas aeruginosa | – ve | – ve | – ve | + ve |
| Salmonella Typhi | – ve | + ve | – ve | – ve |
| Salmonella Paratyphi A | – ve | + ve | – ve | – ve |
| Salmonella Typhimurium | – ve | + ve | – ve | + ve |
| Citrobacter freundii | – ve | + ve | – ve | + ve |
| Citrobacter koseri | + ve | + ve | – ve | + ve |
| Enterobacter cloacae | – ve | – ve | + ve | + ve |
| Enterobacter aerogenes | – ve | – ve | + ve | + ve |
| Shigella flexneri | Variable | + ve | – ve | – ve |
| Shigella dysenteriae | Variable | + ve | – ve | – ve |
| Proteus vulgaris | + ve | + ve | – ve | (11 – 25% +ve) rest – ve |
| Proteus mirabilis | – ve | + ve | – ve | + ve |
| Serratia marcescens | – ve | – ve | + ve | + ve |
| Morganella morganii | + ve | + ve | – ve | – ve |
| Yersinia enterocolitica | (26 – 75% + ve) | + ve | – ve | – ve |
| Yersinia pestis | – ve | + ve | – ve | – ve |
| Vibrio cholerae | + ve | – ve | Variable | Variable |
| Vibrio parahaemolyticus | + ve | – ve | – ve | – ve |
| Staphylococcus aureus | – ve | + ve | + ve | + ve |
| Staphylococcus epidermidis | – ve | – ve | + ve | – ve |
| Staphylococcus saprophyticus | – ve | – ve | + ve | – ve |
Uses of IMViC Test
- It is routinely used in clinical, research, and teaching laboratory to characterize and identify unknown isolated bacteria up to the level of genus.
- Alongside the Urease test and TSI (Triple Sugar Iron) test, the test can differentiate and identify the members of the Enterobacteriaceae family.
- Some tests are used to differentiate species of a genus. E.g., the indole test is used to differentiate K. oxytoca and K. pneumoniae, C. koseri and C. freundii, P. vulgaris and P. mirabilis, etc.
Limitations of IMViC Test
- It is not enough to completely identify the bacteria up to the level of species. Additional tests are required, even for differentiating Enterobacterales.
- Different genera give the same results, hence results become ambiguous.
- It is culture-based test series and hence requires a longer period. Some bacteria require more than 2 days of incubation to show VP reaction, and for the citrate test, we may have to incubate for more than 4 days.
- Only the culturable bacteria can be characterized.
- The whole process is complex and requires good culture skills and various resources to do the procedure.
- False positive results and false negative results are encountered if the incubation period is not appropriate and the reagents are old or not used appropriately.
References
- Powers, E. M., & Latt, T. G. (1977). Simplified 48-hour IMVic test: an agar plate method. Applied and environmental microbiology, 34(3), 274–279. https://doi.org/10.1128/aem.34.3.274-279.1977
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3. DOI:10.1128/9781555818814.ch3.17.1
- MacWilliams, M. P. (2009, December 8). Indole Test Protocol. https://www.asmscience.org/content/education/protocol/protocol.3202
- Color Atlas and Textbook of Diagnostic Microbiology, Koneman, 5th edition
- Bailey & Scott’s Diagnostic Microbiology. Editors: Bettey A. Forbes, Daniel F. Sahm & Alice S. Weissfeld, 12th ed 2007, Publisher Elsevier.
- Colour Atlas and Textbook of Diagnostic Microbiology. Editors: Koneman E.W., Allen D.D., Dowell V.R. Jr, and Sommers H.M.
- Mackie and Mc Cartney Practical Medical Microbiology. Editors: J.G. Colle, A.G. Fraser, B.P. Marmion, A. Simmous, 4th ed, Publisher Churchill Living Stone, New York, Melborne, Sans Franscisco 1996.
- Monica Cheesbrough Distinct Laboratory Practice in Tropical Countries…2nd Edition
- Hansen DS, Aucken HM, Abiola T, Podschun R. Recommended test panel for differentiation of Klebsiella species on the basis of a trilateral interlaboratory evaluation of 18 biochemical tests. J Clin Microbiol. 2004 Aug;42(8):3665-9. doi: 10.1128/JCM.42.8.3665-3669.2004. PMID: 15297514; PMCID: PMC497635.
- Jones JL, Lüdeke CH, Bowers JC, Garrett N, Fischer M, Parsons MB, Bopp CA, DePaola A. Biochemical, serological, and virulence characterization of clinical and oyster Vibrio parahaemolyticus isolates. J Clin Microbiol. 2012 Jul;50(7):2343-52. doi: 10.1128/JCM.00196-12. Epub 2012 Apr 25. PMID: 22535979; PMCID: PMC3405591.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2130244/pdf/jhyg00067-0137.pdf
- IMVIC Test | Microbiology | Biotechnology Methods | Botany Laboratory Experiments | Biocyclopedia.com
- IMViC Tests: Principle, Procedure, Results • Microbe Online
- Biochemical Test (IMViC) In Microbiology – Solution Pharmacy
- IMViC Tests : Principle, Procedure and results – Lab Tests Guide
- 37: IMViC tests – Biology LibreTexts
- IMViC test: Introduction, Principle, Test Procedure and Result Interpretation (universe84a.com)
- IMViC tests: Principle, Procedure and Results, Notes & MCQ For Microbiology Exams – Gpatindia: Pharmacy Jobs, Admissions, Scholarships, Conference,Grants, Exam Alerts
- https://bioinfo.bisr.res.in/cgi-bin/project/docter/get_details.cgi?query=all&&organism=proteus&&species=Proteus%20vulgaris

Thank you for this valuable effort, greatly benefiting students and ensuring accessibility for all by making it 🆓. Again thank you by heart
Very useful! How do I reference it?
Thank you.
You can cite as below:
Dahal, P. (2022) IMVIC test- principle, result chart, examples, uses, Microbe Notes. Available at: https://microbenotes.com/imvic-tests/ (Accessed: April 3, 2023).
The article is very oky ….
Thank you for providing the citation. Very useful indeed. Bravo
It’s so use full .. thank you