Enterococcus faecalis is a Gram-positive, catalase-negative, non-motile cocci of the genus Enterococcus in the Enterococcaceae family of the Lactobacillales order in the class Bacilli.
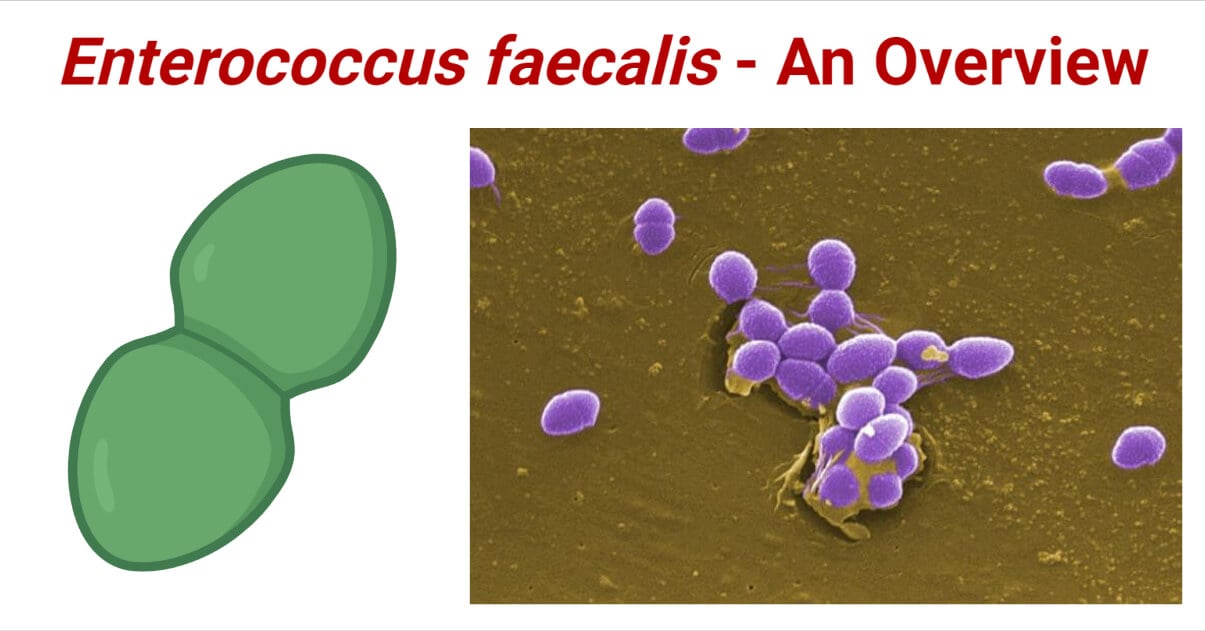
- It is a commensal bacterium found in the GI tract (gastrointestinal tract) of humans. It is also found in the vaginal tract of some females as commensal. However, it is frequently reported in several nosocomial infections including urinary tract infections, wound and soft-tissue infections, bloodstream infections, endocarditis, meningitis, etc.
- Enterococcus faecalis was first isolated and identified in 1899 by a German microbiologist named Fritz Schmalz. Due to its similarities to other Streptococcus bacteria at the time, it was categorized as Streptococcus faecalis. E. faecalis was once categorized as a member of the Group D Streptococci, which were distinguished by their capacity to thrive in bile.
- According to a paper written by the International Council of Microbiologists in 1984, the “Streptococcus faecalis group” of bacteria should be reclassified. This reclassification was made in light of many considerations, such as variations in biochemical characteristics and cell wall structure, as well as genetic data from DNA hybridization investigations. Given that these bacteria are frequently found in the gastrointestinal tracts of both people and animals, the name “Enterococcus”, (derived from the Greek words “enteron,” which means intestine, and “kokkos,” which means berry or coccus) was suggested. As a result, the Streptococcus faecalis group was reclassified as a distinct genera ‘Enterococcus’ and the species S. faecalis was reclassified as Enterococcus faecalis.
- Recently, it is part of a larger group the ‘Enterococcaceae family’, and has become a major concern in healthcare settings due to its resistance to numerous antibiotics and ability to cause a wide range of nosocomial infections.
Interesting Science Videos
Classification of Enterococcus faecalis
Formally, ‘Enterococcus faecalis’ was classified in the Streptococcaceae family under Group D Streptococcus group. Since, the 1980s, the classification was changed and Group D Streptococci were reclassified into a separate family ‘Enterococcaceae’ and the name of the bacterium Streptococcus faecalis was changed to Enterococcus faecalis. Besides, there is another controversy in the name of the phylum; modern microbiologists suggest using the new name ‘Firmicutes’ while some still use the old name ‘Bacillota’.
| Domain | Bacteria |
| Phylum | Firmicutes (Previously Bacillota) |
| Class | Bacilli |
| Order | Lactobacillales |
| Family | Enterococcaceae |
| Genus | Enterococcus |
| Species | E. faecalis |
Habitat of Enterococcus faecalis
- E. faecalis is able to adapt and survive in a range of environments and with varied conditions; however, its major habitat is the intestinal tract of warm-blooded animals including humans. It is also found to colonize the genitourinary tract (especially the vaginal tract), oral cavity, and skin.
- In nature, it is also found in soil, water, food, and decaying vegetation. It is abundantly found in fecal-contaminated soil and water.
Morphology of Enterococcus faecalis
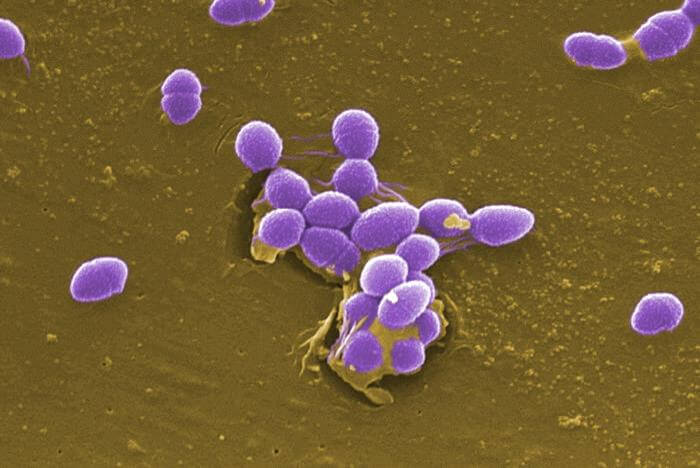
- E. faecalis are Gram-positive cocci appearing slightly oval-shaped under higher magnification. The cells are typically 0.5 to 2 μm in diameter and elongate up to 0.6 to 2.5 μm under some growth conditions giving an ovoid appearance.
- Under the microscope, they are seen as oval in shape which may be either single, arranged in pairs, or chains.
- They are non-motile, non-spore-forming, non-pigment-producing bacteria. They can develop pili and form biofilm which help them to colonize and survive in harsh conditions.
Cultural Characteristics of Enterococcus faecalis
- E. faecalis is non-fastidious bacterial species; hence, it can be grown on a wide range of general-purpose as well as selective culture media. They are facultative anaerobes and produce visible colonies in the temperature range from 100C to 450C. They are resistant to conditions like 6.5% NaCl concentration, bile salts, higher pH up to 9.5, and even temperature of 600C for about half an hour. Despite these characteristics, E. faecalis is commonly grown aerobically at 35 (±2)0C.
- Blood agar medium is routinely used for primary isolation and characterization of E. faecalis in a microbiology lab. Other media like Nutrient Agar, Bile Esculin Agar, Columbia CNA medium, and MacConkey Agar are also used for their isolation and identification.
- There is some selective medium for isolation and rapid primary identification of Enterococcus spp. including E. faecalis such as CHROMagarTM Orientation, Brilliance UTI Agar, Enterococcosel agar, and mEnterococcus Agar (Slanetz and Bartley agar).
Cultural characteristics of Enterococcus faecalis in some of the commonly used culture media are described below:
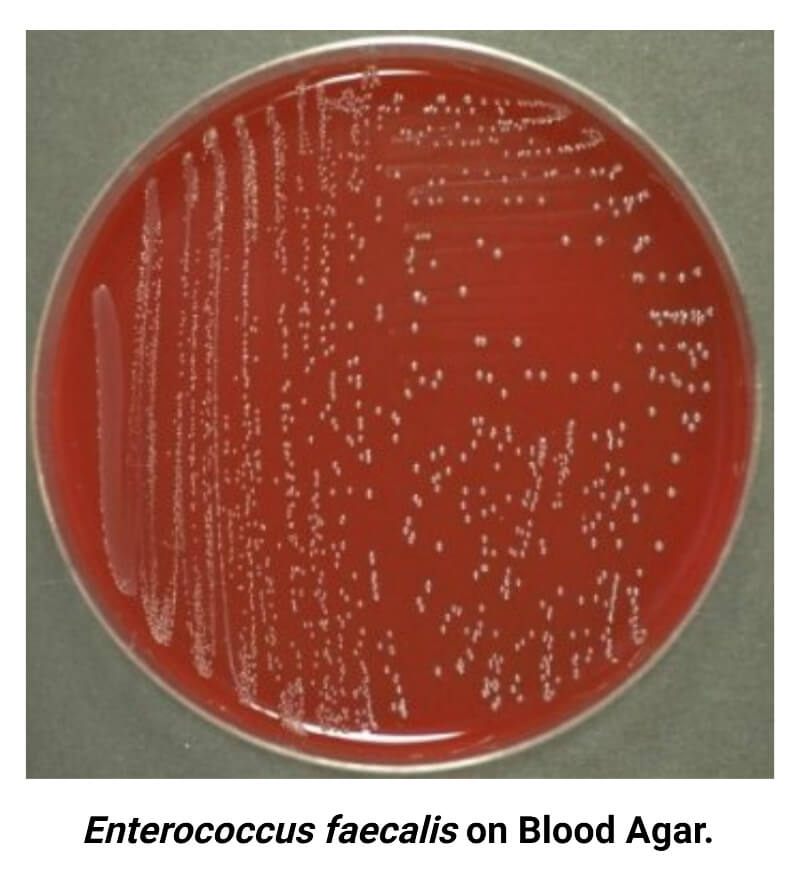
- Blood Agar Medium
Colonies of E. faecalis are small (pinpoint colonies), smooth, gray or grayish-white, non-hemolytic (γ- hemolytic) in appearance. Rarely, some strains produce a narrow zone of β- or α- hemolysis around colonies.
- Bile Esculin Agar Medium
E. faecalis is a bile salt tolerant and can grow in 40% bile salt concentration. E. faecalis develop small, convex, transparent slightly brownish colonies with a brown-black halo around colonies. They may even cause the blackening of the medium.
- Nutrient Medium
On nutrient agar, E. faecalis produces small, spherical, smooth, opaque, creamy colonies.
- MacConkey Agar Medium
On MAC agar medium without crystal violet, E. faecalis produces small, smooth colonies with pin color indicating its ability to ferment lactose.
- Columbia CNA Agar Medium
Columbia CNA agar medium is a selective medium for Gram-positive cocci. In this medium, E. faecalis produces small, convex, mostly non-hemolytic, opaque circular colonies with slight bluish coloration in some cultures.
- Brilliance UTI Agar
It is a chromogenic differential medium used to isolate and rapidly presume bacterial pathogens associated with urinary tract infections (UTIs). In Brilliance UTI agar, E. faecalis produces small, circular, smooth colonies with turquoise blue color (blue-green color).
- CHROMagarTM Orientation
In CHROMagarTM Orientation, E. faecalis develop small circular colonies of turquoise blue color.
- M-Enterococcus Medium (Slanetz and Bartley Agar)
It is a selective medium primarily used for the selective isolation of Enterococcus spp. from water and other food products. In this medium, E. faecalis produces small, circular, smooth, convex colonies of pink or white color.
Biochemical Characteristics of Enterococcus faecalis
General Biochemical Test Results
| General Biochemical Characteristics | Enterococcus faecalis |
| Arginine dihydrolase Test | Positive (+) |
| 40% Bile-salt Tolerance | Positive (+) |
| Bile Solubility Test | Insoluble |
| Capsule | Negative (-) |
| Catalase | Negative (-) |
| Citrate (Simmons) | Negative (-) |
| Coagulase | Negative (-) |
| Deoxyribonuclease (DNase) | Negative (-) |
| Esculin Hydrolysis | Positive (+) |
| Gram Staining | Gram Positive Cocci |
| Gas (in MRS broth) | Negative (-) |
| Hemolysis | γ- hemolytic (rarely α-and β-) hemolytic |
| H2S (Hydrogen Sulfide) | Negative (-) |
| LAP (Leucine amino peptidase) Test | Positive (+) |
| Motility | Non-motile |
| 6.5% NaCl | Positive (+) |
| Nitrate Reduction | Positive (+) |
| Oxidase | Negative (-) |
| OF (Oxidative Fermentation) | Facultative Anaerobes |
| PYR (Pyrrolidonyl Aryl amidase) Test | Positive (+) |
| Pyruvate Broth Test | Positive (+) |
| Spore | Negative (-) |
| Growth at 4°C Growth at 10°C Growth at 40°C | Negative (-) Positive (+) Positive (+) |
Carbohydrate Fermentation Tests
| General Biochemical Characteristics | Enterococcus faecalis |
| N-Acetylglucosamine | Positive (+) |
| Adonitol | Negative (-) |
| L-Arabinose | Negative (-) |
| D-Arabitol | Negative (-) |
| L-Arabitol | Negative (-) |
| Arbutin | Negative (-) |
| Cellobiose | Positive (+) |
| Dulcitol | Negative (-) |
| D-Fructose | Positive (+) |
| D- and L-Fucose | Negative (-) |
| Galactose | Positive (+) |
| D-Glucose | Positive (+) |
| Glycerol | Positive (+) |
| Glycogen | Negative (-) |
| Inulin | Negative (-) |
| Lactose | Positive (+) |
| Maltose | Positive (+) |
| Mannitol | Positive (+) |
| D-Mannose | Positive (+) |
| D-Raffinose | Negative (-) |
| Rhamnose | Variable |
| Ribose | Positive (+) |
| Sorbitol | Positive (+) |
| Sorbose | Negative (-) |
| Sucrose | Positive (+) |
| D-Tagatose | Positive (+) |
| Trehalose | Positive (+) |
| Tartrate | Negative (-) |
| L-Xylose | Negative (-) |
| D-Xylose | Variable |
Enzymatic Hydrolysis Tests
| General Biochemical Characteristics | Enterococcus faecalis |
| Arginine Dehydrolase | Positive (+) |
| Caseinase | Negative (-) |
| DNase | Negative (-) |
| Esculin Hydrolysis | Positive (+) |
| α-Galactosidase | Negative (-) |
| Gelatinase | Positive (+) |
| Lipase | Positive (+) |
| Lysine Decarboxylase | Negative (-) |
| Leucine Arylamidase | Positive (+) |
| Ornithine Decarboxylase | Negative (-) |
| Phenylalanine Deaminase | Negative (-) |
| Pyrrolidonyl Aminopeptidase | Positive (+) |
| Tryptophanase | Positive (+) |
Virulence Factors of Enterococcus faecalis
Though E. faecalis is a bacterium of concern, especially for nosocomial infections, little is known about its virulence factors. Some well-known virulence factors are described below:
1. Adhesins
These are the surface-associated proteins or structures that facilitate the attachment of E. faecalis cells to the host’s cells or tissues. E. faecalis produces several adhesins like pili (fimbriae), collagen-binding adhesins, enterococcal surface proteins (ESPs), aggregation substances (Agg), etc. These adhesins allow them to attach to the host’s surface, develop biofilm, promote colonization, and protect from flushing action inside the urinary tract.
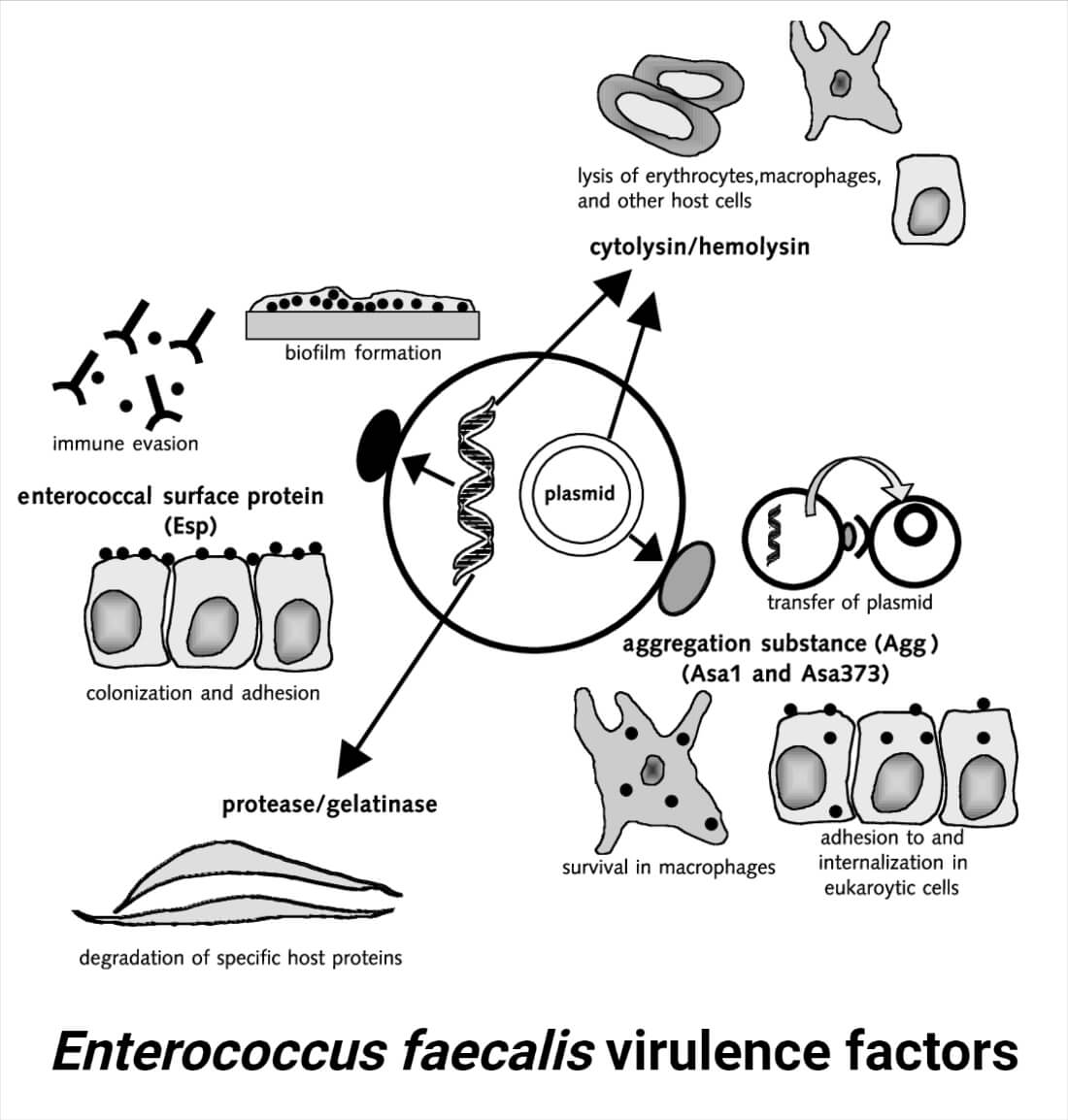
2. Hemolysin/Cytolysin
E. faecalis is known to produce hemolysin which is responsible for the lysis of the host’s cells. This protein helps the bacterium to invade the host’s tissue, evade immune cells, and damage the host’s tissues.
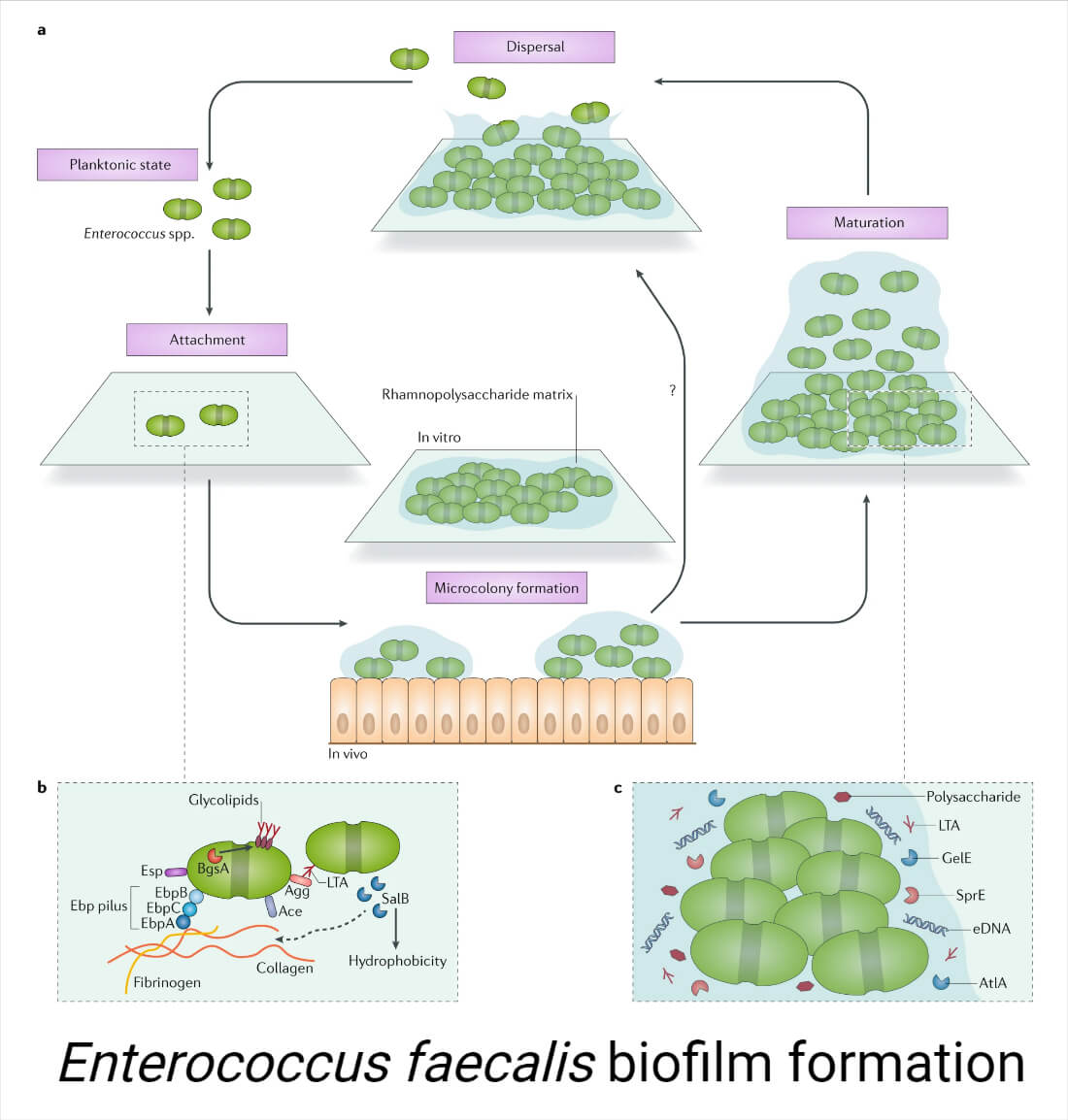
3. Biofilm Formation
Biofilm formation is one important virulence factor of E. faecalis. The formation of biofilm allows the bacterium to escape the immune system, protect against changing environments and antibiotic components, and colonize several surfaces like medical devices and host tissues.
4. Gelatinase
This extracellular enzyme produced by E. faecalis helps in the destruction of extracellular matrix components and the host’s tissue. This facilitates the spread of the bacteria inside a host, the acquisition of essential nutrients for the bacteria, and the invasion of the host’s tissues.
Pathogenesis of Enterococcus faecalis Infections
E. faecalis is a commensal and opportunistic pathogen causing different types of infections including UTIs, soft tissue infections, wounds, bloodstream infections, endocarditis, etc. Virulence factors like adhesins, extracellular enzymes, biofilm-forming ability, antimicrobial resistance, etc. support its pathogenesis. Although the pathogenesis of the disease differs from type and site of infection, a general step of the pathogenesis of E. faecalis infection can be summarized as:
- Adherence and Colonization
E. faecalis adhere to the host’s cell surface using several adhesins described above. Once adhered, they multiply rapidly colonizing the adhered site.
- Biofilm Formation
E. faecalis can develop biofilm over catheters and other implanted medical devices and on the host’s tissue providing protection to the bacteria against immune response and antimicrobials and supporting persistent infection.
- Release of Toxins and Extracellular Enzymes
Once adhered and colonized, the bacterium releases toxins and extracellular enzymes like hemolysin, gelatinase, lipase, proteases, etc. These released proteins allow E. faecalis to destroy the host’s tissue component and disseminate and survive.
- Invasion and Evasion
The released toxins and enzymes allow them to invade the host’s epithelial cells and evade the immune responses.
- Host’s Immune Response (Inflammatory Response)
The presence of the bacterium and the destruction of cells trigger the inflammatory responses and other immune responses within the host’s body. The inflammation recruits neutrophils and macrophages at the infected site and further damages the tissue causing swelling, pain, watery discharge, etc.
Clinical Manifestation of Enterococcus faecalis
E. faecalis demonstrate a wide range of clinical manifestations in humans. They are typically reported in healthcare-associated infections and rarely associated with community-acquired infections. E. faecalis are opportunistic pathogens meaning they mainly infect persons with compromised or weak immune systems. Some common diseases caused by E. faecalis in humans are listed below:
- Urinary Tract Infections (UTIs)
UTI is the most common clinical manifestation of E. faecalis. It is generally reported in healthcare-associated UTIs, especially in catheterized and immunocompromised patients. They commonly cause cystitis and pyelonephritis.
- Intra-abdominal Infections
Despite being commensals of the human gastrointestinal tract, E. faecalis cause intra-abdominal infections like peritonitis and intestinal abscesses formation. In fact, E. faecalis is found to be associated with about 30% of cases of intra-abdominal infections (Fabre, V. et.al, 2019).
- Endocarditis
Being responsible for 5% to 15% of cases, Enterococcus faecalis is the third most common cause of infective endocarditis (Seby, R. et. al, 2022). It is mainly caused due to dissemination of bacteria through the bloodstream after infecting the genitourinary tract.
- Bloodstream Infections
E. faecalis-associated bacteremia and sepsis in many cases. Mostly the infections are nosocomial, but community-acquired bloodstream infection by E. faecalis is also frequently reported.
- Surgical Site Infections
E. faecalis can produce infections at the surgical site after procedures, which can result in wound infections, the development of abscesses, or deep tissue infections.
- Rarely E. faecalis is also associated with ocular infections, respiratory infections, and nervous system infections like meningitis.
Laboratory Diagnosis of Enterococcus faecalis
In the laboratory, E. faecalis is mainly isolated from samples like urine and pus/swab or any other clinical samples from infected sites. There is no need for special consideration for sample collection, transportation, and processing.
Identification is mainly based on combined results of the study of cultural characteristics, microscopic observation, and biochemical tests. Molecular diagnosis is also available for accurate identification and molecular or genetic characterization.
1. Cultural Characters and Morphological Identification
Commonly, blood agar medium (BA), Columbia CNA agar medium, and Bile Esculin Agar medium are used in a laboratory for the culture of samples suspected with Enterococcus spp. The appearance of colonies in any of these mediums or any other used culture medium is reported and compared with colonial characters of E. faecalis in the medium.
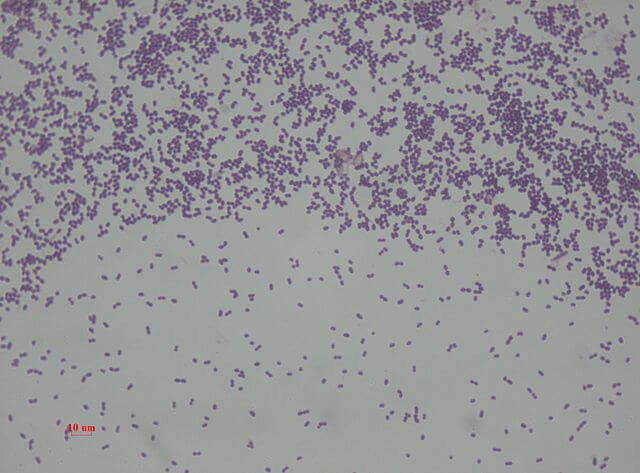
On microscopic observation after Gram-staining, they appear as Gram-positive cocci with elongated oval-shaped cells, generally arranged in pairs or chains.
2. Biochemical Characterization
Once the bacteria are confirmed to be Gram-positive cocci, a series of biochemical tests is used to confirm it as E. faecalis. Tests commonly used are catalase test, oxidase test, LAP test, PYR test, Bile Esculin test, growth on 6.5% NaCl, arginine dihydrolase test, and carbohydrate (pyruvate, mannitol, and raffinose) fermentation tests.
3. Molecular Diagnosis
For more rapid and accurate identification, molecular diagnostic tools like PCR and sequencing, rRNA sequencing, and Mass spectrometry using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF MS), etc. are preferred. However, in clinical diagnosis, these methods are rarely used.
Treatment of Enterococcus faecalis Infections
Tetracycline, nitrofurantoin, quinolones, ampicillin, and tetracycline are all used for UTIs. Penicillin (Penicillin-G), ampicillin, or vancomycin together with an aminoglycoside like gentamycin or streptomycin are frequently given for systemic deadly infections. Linezolid and daptomycin, two more recent antimicrobials, are also occasionally utilized. Chloramphenicol and other antibiotics are occasionally used to treat MDR-E. faecalis infections. Vancomycin is used against beta-lactamase-producing E. faecalis strains. However, due to the development of antimicrobial resistance against them by E. faecalis, a number of these antibiotics are becoming less or ineffective. Currently, combination therapy including cell-wall active agents (penicillin, ampicillin, or vancomycin) and an aminoglycoside is widely used against E. faecalis infections.
(References: Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Bailey and Scott’s Diagnostic Microbiology. 12th Edition)
Antibiotic Resistance Profile of Enterococcus faecalis
- Enterococci have intrinsic and inherited resistance to a wide range of commonly used antimicrobial agents like Aminoglycosides, Beta-lactams, and Glycopeptides. Resistant to all the used therapeutics, including newer ones like linezolid, daptomycin, and vancomycin, have been reported in several reports. This antimicrobial-resistant profile has made it very difficult to treat the infections of E. faecalis using single antibiotics; hence, demanded combination therapy and need for long-term antibiotic consumption.
- E. faecalis is intrinsically resistant to several penicillin derivatives. Though they were initially sensitive to ampicillin, the acquisition of beta-lactamase-producing genes and alterations in their penicillin-binding proteins (PBPs) has rendered a reduction in the efficiency of ampicillin when used singly or in combination.
- Similarly, the production of aminoglycoside-modifying enzymes, rRNA methylase enzymes, formation of efflux pumps, and change in DNA gyrase and topoisomerase-IV has made E. faecalis resistant against aminoglycosides, macrolides, tetracyclines, and quinolones respectively.
Vancomycin-Resistant Enterococci (VRE)/Vancomycin Resistant E. faecalis (VRE)
Vancomycin is the reserved antibiotic for the treatment of E. faecalis and it is the drug of choice for beta-lactam-resistant and aminoglycoside-resistant strains. However, in recent years, resistance against vancomycin by E. faecalis has been globally reported.
The main mechanism of resistance in E. faecalis is the acquisition of resistance genes, most frequently vanA or vanB genes. These genes can be transferred between various bacterial strains and species because they are frequently carried on movable genetic components known as plasmids or transposons.
The extensive use of vancomycin, especially in healthcare settings, has put selective pressure on the development of VRE. Infection by VRE strains is a severe threat and cause for concern due to the restricted treatment choices for VRE. These infections typically require combination therapy or newer antibiotics like linezolid and daptomycin, which raises the cost of treatment.
Prevention of Enterococcus faecalis Infections
E. faecalis is an opportunistic pathogen and is mainly involved in healthcare-associated infections. Procedures such as hand hygiene, correct equipment disinfection, environmental cleaning, and isolation of infected or colonized individuals, are necessary to stop the spread of E. faecalis. Strict infection control procedures, surveillance systems, and antibiotic stewardship programs must be implemented in healthcare settings.
References
- Jett, B. D., Huycke, M. M., & Gilmore, M. S. (1994). Virulence of enterococci. Clinical Microbiology Reviews, 7(4), 462-478. https://doi.org/10.1128/cmr.7.4.462
- Manero, A., & Blanch, A. R. (1999). Identification of Enterococcus spp. with a Biochemical Key. Applied and Environmental Microbiology, 65(10), 4425-4430. https://doi.org/10.1128/aem.65.10.4425-4430.1999
- Murray, PR; Baron; JH; Jorgensen; et al. Manual of Clinical Microbiology, 9th ed. Enterococcus species, Chapter 62. American Society for Microbiology Press, 2007.
- Sherris Medical Microbiology, An Introduction to Infectious Diseases, 4th ed. Chapter 17, Streptococci and Enterococci.
- Deibel, R. H. (1964). UTILIZATION OF ARGININE AS AN ENERGY SOURCE FOR THE GROWTH OF STREPTOCOCCUS FAECALIS. Journal of Bacteriology, 87(5), 988-992. https://doi.org/10.1128/jb.87.5.988-992.1964
- Tendolkar PM, Baghdayan AS, Shankar N. Pathogenic enterococci: new developments in the 21st century. Cell Mol Life Sci. 2003 Dec;60(12):2622-36. doi: 10.1007/s00018-003-3138-0. PMID: 14685687.
- Shankar, N., Baghdayan, A. S., & Gilmore, M. S. (2002). Modulation of virulence within a pathogenicity island in vancomycin-resistant Enterococcus faecalis. Nature, 417(6890), 746–750. https://doi.org/10.1038/nature00802
- Fabre, V., Nemati, K., Avdic, E., Cosgrove, S. E., Amoah, J., & Tamma, P. D. (2019). The Role of Ertapenem for the Treatment of Complicated Intra-abdominal Infections With a Positive Culture for Enterococcus faecalis. Open Forum Infectious Diseases, 6(1). https://doi.org/10.1093/ofid/ofy339
- Seby, R., Kim, C., Khreis, M., & Khreis, K. (2022). Enterococcus faecalis-induced infective endocarditis: An unusual source of infection and a rare clinical presentation. The Journal of International Medical Research, 50(7). https://doi.org/10.1177/03000605221112019
- Cattaneo, C., Rieg, S., Schwarzer, G., Müller, M. C., Blümel, B., & Kern, W. V. (2021). Enterococcus faecalis bloodstream infection: Does infectious disease specialist consultation make a difference? Infection, 49(6), 1289-1297. https://doi.org/10.1007/s15010-021-01717-3
- Forbes BA, Sahm DF, Weissfeld AS. Bailey & Scott’s Diagnostic Microbiology, 12th ed. Mosby Elsevier, 2007. Chapter 20, Enterococcus, pp. 259-271
- Connie R. Mahon, Donald C. Lehman, and George Manuselis. Textbook of Diagnostic Microbiology, 5th ed.
- Kim, M., Rosa, V., & Min, K. (2020). Characterization of Enterococcus faecalis in different culture conditions. Scientific Reports, 10(1), 1-8. https://doi.org/10.1038/s41598-020-78998-5
- Anderson, A. C., Jonas, D., Huber, I., Karygianni, L., Wölber, J., Hellwig, E., Arweiler, N., Vach, K., Wittmer, A., & Al-Ahmad, A. (2015). Enterococcus faecalis from Food, Clinical Specimens, and Oral Sites: Prevalence of Virulence Factors in Association with Biofilm Formation. Frontiers in Microbiology, 6. https://doi.org/10.3389/fmicb.2015.01534
- Repoila, F., Le Bohec, F., Guérin, C., Lacoux, C., Tiwari, S., Jaiswal, A. K., Santana, M. P., Kennedy, S. P., Quinquis, B., Rainteau, D., Juillard, V., Furlan, S., Bouloc, P., Nicolas, P., Miyoshi, A., Azevedo, V., & Serror, P. (2022). Adaptation of the gut pathobiont Enterococcus faecalis to deoxycholate and taurocholate bile acids. Scientific Reports, 12(1), 1-15. https://doi.org/10.1038/s41598-022-12552-3
- Biochemical Test and Identification of Enterococcus faecalis (microbiologyinfo.com)
- ENTEROCOCCUS FAECALIS: CHARACTERISTICS, MORPHOLOGY, PATHOGENESIS – BIOLOGY – 2023 (sperohope.com)
- Enterococcus faecalis – microbewiki (kenyon.edu)
- Enterococcus faecalis: Properties, Pathogenesis, Lab Diagnosis • Microbe Online
- E. faecalis: Infections, transmission, treatment, and prevention (medicalnewstoday.com)
- Enterococcus Faecalis: Symptoms, Causes, Treatment (verywellhealth.com)
- Enterococcus Faecalis: Causes, Symptoms, and Treatments (healthline.com)
- Enterococcus faecalis. (2023, May 10). In Wikipedia. https://en.wikipedia.org/wiki/Enterococcus_faecalis
- Enterococcus faecalis – an overview | ScienceDirect Topics
- https://universe84a.com/enterococcus-identification/
- Taxonomy browser (Enterococcus faecalis) (nih.gov)
- https://www.chromagar.com/en/product/chromagar-orientation/
- https://www.dalynn.com/dyn/ck_assets/files/tech/PB65.pdf
- https://assets.fishersci.com/TFS-Assets/MBD/Instructions/Brilliance-UTI-User-Guide-PO0794A-PO5120A-PO110A-PO5159A-EN.pdf
