Although most bacteria are able to ferment (metabolize) glucose, the end product of glucose metabolism by different bacteria might not always be the same. Following glycolysis, some follow the mixed acid fermentation pathway to convert pyruvate into a stable organic acid mixture, while others follow the butylene glycol pathway to produce acetylmethylcarbinol (acetoin) and butanediol.
VP test is a biochemical test that detects the ability of bacteria to metabolize the pyruvate into a neutral intermediate product called ‘acetylmethylcarbinol’ or ‘acetoin’.
It is a part of the IMViC test series, a set of four biochemical tests used for the differentiation and identification of Gram-negative bacteria, especially Enterobacterales.
This test was first described by Daniel W. O. Voges and Bernhard Proskauer in 1898. The test was modified several times and it was only in 1936 that Barritt suggested the use of α-naphthol and KOH to get more vivid and intensified results. Today, the Barritt-modified VP test is widely used for the characterization of Gram-negative bacilli and Actinobacteria.
Interesting Science Videos
Objectives of VP Test
- To differentiate bacteria based on their property to produce acetoin as the end product of glucose fermentation
- To characterize and differentiate Gram-negative bacilli (primarily Enterobacterales) and Actinobacteria
Principle of VP Test
After glycolysis, the produced pyruvate can be metabolized through the butylene glycol pathway producing two neutral end products, acetylmethylcarbinol (also known as acetoin) and butanediol.
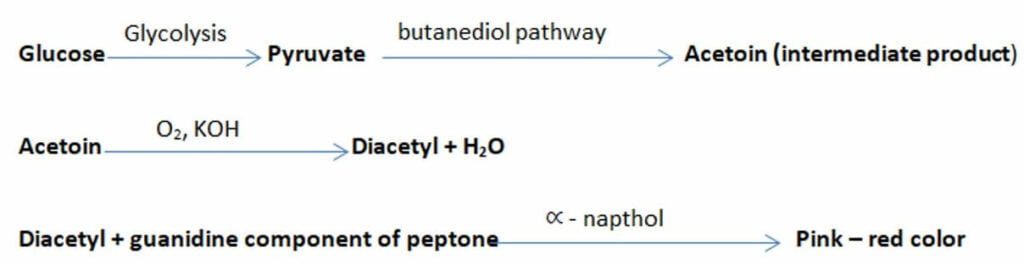
- Thus produced acetoin will be oxidized to diacetyl in the presence of KOH (potassium hydroxide) and air (O2). Thus produced diacetyl, in the presence of ∝ – naphthol, will react with the guanidine component of peptone forming a pink to red colored polymer.
Requirements for VP Test
1. Culture Media
The MR-VP broth, also known as Glucose Phosphate Broth, is used for performing the VP test.
Composition of MR-VP Broth per 1000 ml
Buffered Peptone – 7.00 grams
Dextrose (Glucose) – 5.00 grams
Dipotassium Phosphate – 5.00 grams
Final pH – 6.9±0.2 at 25°C
Preparation of MR-VP Broth
- Measure the appropriate amount of MR-VP broth media powder (17.0 grams per 1000 mL) and dissolve it in the water of the required volume in a conical flask (or glass bottle).
- Stir well in a magnetic stirrer or manually and heat (if necessary) to completely dissolve the media powder.
- Dispense about 5 to 10 mL of the broth in a clean test tube and loosely put it on the cap or cotton plug.
- Autoclave the test tubes at 121°C and 15 lbs pressure for 15 minutes and let them cool to below 40 – 45°C before inoculation.
2. Reagents
5% Alpha-naphthol Solution (Barritt’s Reagent A) and 40% KOH or NaOH solution (Barritt’s Reagent B) are required.
Preparation of Barritt’s Reagent A
Dissolve 5 grams of α-naphthol reagent in 100 mL of 95% ethanol. The reagent can be stored for up to 3 weeks in a dark place at 4 to 8°C.
Preparation of Barritt’s Reagent B
Dissolve 40 grams of KOH pellet in 100 mL of sterile distilled water. The reagent can be stored for up to 3 weeks at 4 to 8°C.
3. Equipment
| Test tubes Incubator | Dropper Autoclave | Bunsen burner Weighing Machine | Inoculating loop |
4. Test Organism (Sample Bacteria)
5. Control Organisms
Escherichia coli ATCC 25922
Klebsiella pneumoniae ATCC 13883
Procedure of VP Test
- Using a sterile inoculating loop, pick up well-isolated colonies of sample bacteria from 18 to 24 hours old culture and inoculate the broth.
- Incubate the tubes aerobically for 18 to 24 hours at 35±2°C.
- Following incubation, transfer 2 mL of broth to a clean (sterile if possible) test tube.
- Add 6 drops of Reagent A (5% α-naphthol solution) and mix properly by shaking.
- Add 2 drops of Reagent B (40% KOH solution) and mix properly by shaking.
- Observe for the formation of red-pink color at the surface of the medium within 30 minutes. Continuously shake the tube vigorously during the 30-minute waiting period.
- If no color is developed (negative reaction) re-incubate the remaining broth for additional 24 hours and test again.
Result Interpretation of VP Test
- A positive result is indicated by the formation of a pink-red color over the surface of the medium.
- A negative result is indicated by a lack of pink-red color over the surface of the medium or the formation of the copper color.

Quality Control
- Klebsiella pneumoniae ATCC 13883 gives positive results i.e. form pink-red color over the surface of the medium.
- Escherichia coli ATCC 25922 gives negative results i.e. do not form pink-red color over the surface of the medium.
VP Test Results of Some Common Bacteria
| VP Positive Bacteria | VP Negative Bacteria |
| Klebsiella spp., Enterobacter spp., Viridans Streptococci (except S. mitis, and S. vestibularis) Proteus mirabilis, Hafnia spp., Serratia spp., Staphylococcus aureus, Listeria spp., V. cholerae | Escherichia spp., Proteus vulgaris, Citrobacter freundii, Morganella morganii, Shigella spp., Yersinia spp., V. parahaemolyticus |
Precautions
- Over-inoculation results in inhibited bacterial growth; hence, it is better to limit the bacterial inoculum to less than 109 cells per mL of broth.
- Use freshly prepared reagents. If reagents need to be stored, always store them in a dark place.
- Mix the medium and reagent properly by shaking vigorously so that oxygen dissolves properly.
- Wait up to 30 minutes after the addition of reagents while shaking continuously before reporting negative.
- Prohibit over-incubation (incubation more than 3 days) because it can give a weak or false negative reaction.
- An excessive amount of KOH gives a weakly positive reaction, so use the reagents in the appropriate amount.
Applications of VP Test
- To identify and differentiate Gram-negative bacteria, primarily Enterobacteriaceae.
- To differentiate Actinobacteria.
Limitations of VP Test
- It is not a confirmatory test and needs results of other biochemical tests to confirm bacterial identification.
- Sometimes the positive result is obtained after 30 minutes to 1 hour. Reading the result after 1 hour may give a false positive result.
- Reagents must be added in the specified order in a specified amount.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition) . Washington, DC : ASM Press 1752 N St., N.W., [2016] doi:10.1128/9781555818814.ch3.17.33
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.) P. 185 – 187. St. Louis, Missouri: Elsevier
- Barry, A. L., & Feeney, K. L. (1967). Two Quick Methods for Voges-Proskauer Test. Applied Microbiology, 15(5), 1138-1141. https://doi.org/10.1128/am.15.5.1138-1141.1967
- https://microbiologyinfo.com/voges-proskauer-vp-test-principle-reagents-procedure-and-result/
- Voges–Proskauer test. (2023, January 4). In Wikipedia. https://en.wikipedia.org/wiki/Voges%E2%80%93Proskauer_test
- https://microbeonline.com/voges-proskauer-test-principle-procedure-results/#Limitations_of_VP_Test
- https://asm.org/getattachment/0c828061-9d6f-4ae7-aea3-66e1a8624aa0/Methyl-Red-and-Voges-Proskauer-Test-Protocols.pdf
- https://microbenotes.com/voges-proskauer-vp-test/
- https://www.sciencedirect.com/topics/immunology-and-microbiology/voges-proskauer-test
- https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.27%3A_MR-VP_Tests
- https://www.himedialabs.com/media/TD/GM070I.pdf
- https://www.himedialabs.com/media/TD/LQ082.pdf

Just found your site and it is interesting, educative, and easy to cope with. Pls carry me along with useful information and documents on laboratory procedures, food microbiology, staining, biochemical tests, mythology, environmental microbiology, culture media, instrumentation, microscopy, cell biology, molecular biology and basic microbiology
Which bacteria are active positive?