A vector is a substance, usually a piece of DNA that carries a sequence of DNA or other genetic material and introduces it into a new cell.
- Vectors act as vehicles to transfer genetic material from one cell to the other for different purposes like multiplying, expressing, or isolation.
- Vectors are used as a tool in molecular cloning procedures so as to introduce the desired DNA insert into a host cell.
- The DNA insert that is transmitted by a vector is termed recombinant DNA, and the process is also known as recombinant DNA technology.
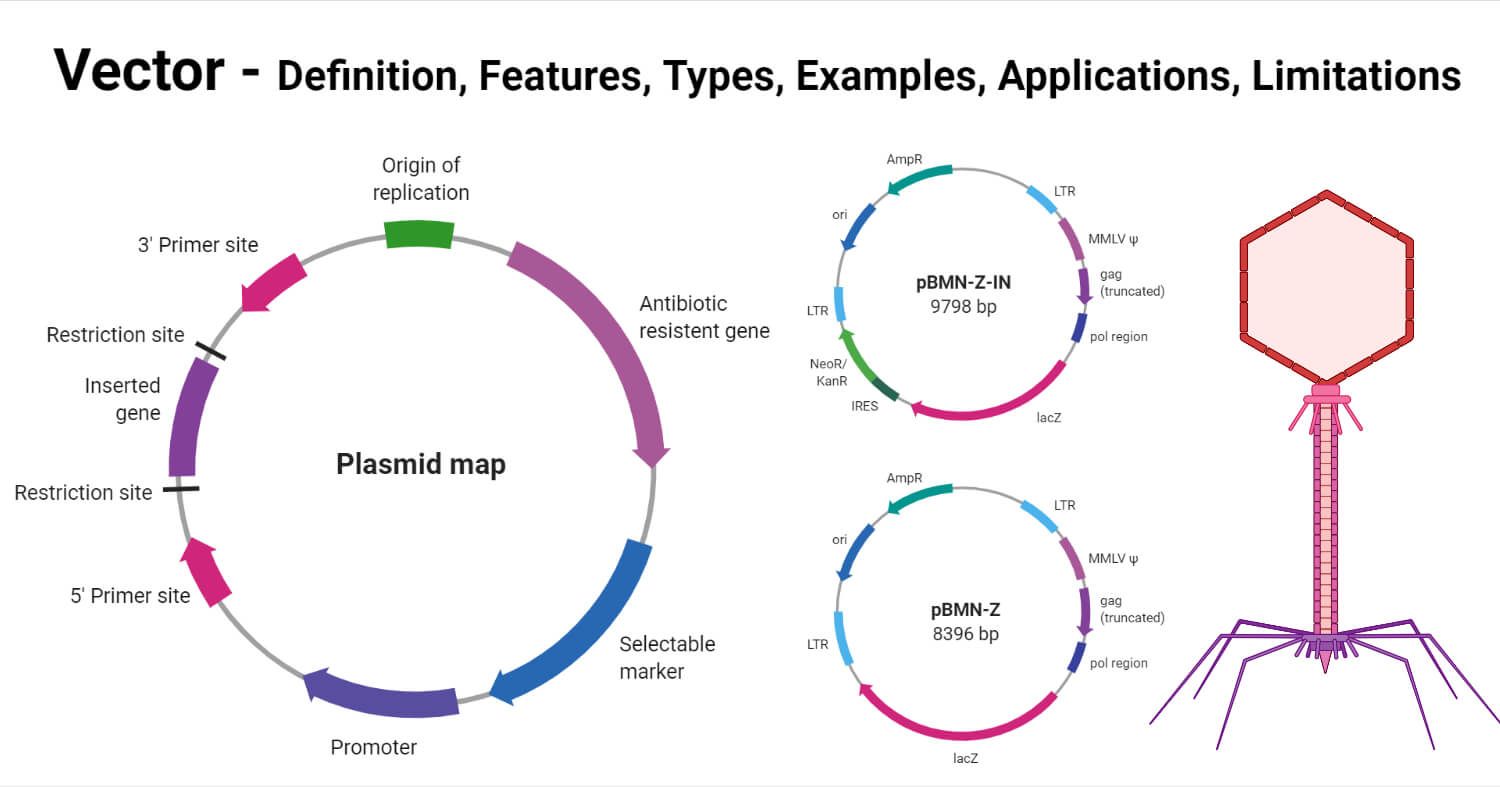
- Usually, the vectors are DNA sequences that carry different parts involved in different functions. Vectors usually have an insert, also known as a transgene, that carries the recombinant DNA and a larger sequence called the backbone of the vector responsible for the structure of the vector.
- Vectors can be classified into different types depending on different characteristics. The selection of vectors thus depends on the purpose of the process.
- Vectors are an important component of the genetic engineering process as these form the basis for the transfer of DNA fragments from one cell to another.
- Vectors have particular features that carry the gene sequences and enable them to survive within the host cell.
- The process of gene transfer also differs in different vectors where some enter the host cell and get incorporated into the host DNA, whereas the others just pass the genetic material into the host cell and recover themselves.
- Even though vectors are usually DNA sequences, viruses and other particles can also function as vectors in processes like transduction.
- Vectors can be reused for multiple processes as these can be recovered at the end of the process.
- A cloning vector is a category of vectors that are essential for cloning procedures. These vectors have different sequences that enable them to initiate replication in host cells as well as propagate within the host.
Interesting Science Videos
Characteristics or Features of vectors
The following are some of the characteristic features of vectors;
- Vectors should be capable of replicating autonomously, which, in turn, depends on the presence of particular sequences in the vector that enables them to initiate replication and propagation within the host cell. Some vectors might even have sequences that allow the production of proteins essential for the inserted DNA, regulation of the process, and further transfer of the insert between different vectors.
- The size of an ideal vector should also be small enough for it to be incorporated into the host genome. The small size of the vector also enables it to incorporate a large-sized insert for transfer.
- Vectors should be easy to isolate and purify as these need to be recovered and reused for multiple processes.
- For a vector to be effective, these should also have certain components that facilitate the process of determining whether the host cell has received the vector. Most vectors used in this process have a gene that either provides resistance to an antibiotic or produces a particular type of protein. These components are called marker genes.
- Many vectors also require unique restriction enzyme recognition sites that enable the insertion of the vector DNA in the presence of specific restriction enzymes. However, many vectors have been designed with a series of restriction sites close to multiple cloning sites that increases the possible restriction enzymes that can be used to digest the sequence.
- The introduction of vectors into the host cell should be easy, which depends on a number of factors.
- In the case of gene transfer processes, it is important that the vector is capable of integrating itself or the recombinant DNA into the genome of the host cell.
- It is important that the introduction of recombinant DNA into the vector doesn’t affect the replication cycle of the vector.
Types of vectors
Vectors can be classified into different groups depending on the purpose of the process and the type of particles used in the process. The following are the commonly studied group of vectors that are used for different purposes;
1. Cloning vectors
- Cloning vectors are vectors that are capable of replicating autonomously and thus are used for the replication of the recombinant DNA within the host cell.
- Cloning vectors are responsible for the determination of which host cells are appropriate for replicating a particular DNA segment.
- Cloning vectors are of further different types that are defined by different features unique to each type of vector.
a. Plasmid vector
- Plasmids are small extrachromosomal circular DNA molecules capable of replicating autonomously within the host cell.
- These are also termed as the workhorse cloning vector in recombinant DNA technology.
- Plasmids are widely used as vectors in all three domains of life; however, these are frequently used in bacteria and yeasts.
- The most important feature of plasmids that makes them one of the best vectors is their small size. The small size of the plasmid facilitates the separation of recombinant DNA from the host’s genomic DNA.
- The size of plasmids ranges from a few thousand base pairs to more than 100 kilobases. The small size of the vector does, however, affect the maximum size of the insert DNA it can carry.
- Plasmids can carry insert DNA that is less than 20 kb as the cloning efficiency and plasmid stability decrease with the size of the vectors.
- The autonomous replication of plasmid is made possible by the presence of genes and sequences that can initiate plasmid replication independent of the host’s replication cycle.
- Bacterial plasmids contain ori sequences that not only control plasmid replication but also determine the possibility of two plasmids coexisting within the same host cell.
- Different plasmids have different types of selective markers, but the most common markers include antibiotic resistance and the production of the β-galactosidase enzyme.
- Some of the most widely used plasmids are pBR322, pUC, and pBluescript vectors that use E. coli as the host.
b. Cosmid
- Cosmid vectors are hybrid vectors composed of plasmid and phage λ vectors, capable of incorporating up to 42 kb of DNA.
- Cosmid vectors are prepared by the insertion of the cos region of the phage vector into the plasmid vectors.
- Cosmid vectors are large-sized vectors with sizes ranging from 400 base pairs to 30 kb. These can carry DNA sequences having sizes ranging from 28 to 46 kb.
- Cosmid vectors are created in order to incorporate large-sized DNA molecules that cannot be carried by plasmids.
- Since these are hybrid vectors, these can replicate within the host cell like plasmids or remain packaged like a phage.
- Cosmid vectors do not have many phage characteristics except the signal sequences that promote phage-head stuffing.
- The hybrid structure of cosmid enables the phage heads to be incorporated within all donor DNA for transfer.
- The use and production of cosmid vectors have increased over the years as the packaged system is highly efficient and selective for the recovery of larger hybrids.
- One of the examples of the cosmid vectors prepared and used in practice are cosmid pHC79 which is a cos-containing derivative of the vector pBR322.
c. Bacteriophage vector
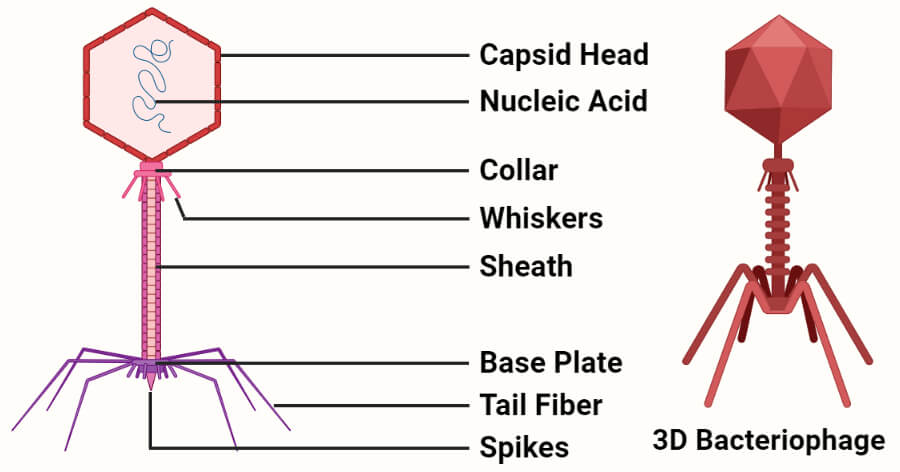
- Bacteriophage vectors are viruses that only infect bacteria and transform them efficiently while carrying large inserts.
- Bacteriophages or phages have higher transformation efficiencies which increase the chances of recovering a clone containing the recombinant DNA segments.
- The most important feature of a phage is the packaging system which enables the incorporation of large eukaryotic genes and their regulatory elements.
- The use of phages also facilitates the isolation of larger quantities of DNA that can be used for the analysis of the insert.
- Even though there are a number of phages that can and have been used as vectors, phage λ is the most convenient cloning vector.
- It can selectively package a chromosome about 50 kb in length, and the size of the phage can be adjusted by removing the central part of the genome as it is not necessary for replication or the packaging of the donor DNA.
- The use of a bacteriophage vector that can incorporate larger DNA segments decreases the number of clones required to obtain a particular DNA library with the entire genome of the organism.
- Phage vectors are also effective as cloning vectors as the recombinant molecules formed after the cloning process are packaged into infective particles that can then be stored or handle efficiently.
- Some of the common phages used as vectors include M13 phages, λ phages, and P1 phages.
d. Bacterial artificial chromosome
- Bacterial artificial chromosomes are engineered DNA molecules that are used to clone DNA segments in bacteria cells (usually E. coli).
- These consist of a bacteria-derived F-factor replication origin which enables the propagation of large DNA fragments in a supercoiled circular form.
- Bacterial artificial chromosomes can carry a much larger size of insert DNA as compared to plasmid or phage vectors.
- These vectors are considered superior over other artificial chromosomes like yeast artificial chromosomes, and mammalian artificial chromosomes as the F-factor found in the bacteria reduces insert chimerism and instability that might arise during the process.
- These are highly efficient as DNA segments as large as 300,000 base pairs can be inserted into bacterial artificial chromosomes, which decreases the number of clones and cycles to be performed to obtain the desired result.
- BAC libraries have been used to generate large genomic DNA inserts for processes like positional cloning, physical mapping, and genome sequencing.
- BAC cloning system has been increasingly used in genetic engineering due to its stability and ease of use as compared to other similar vectors.
- However, BACs have been associated with the random insertion of DNA fragments into the host genome resulting in unpredicted expression.
e. Yeast artificial chromosome
- Yeast artificial chromosomes are engineered DNA molecules that are used to clone DNA inserts within the yeast cells, particularly Saccharomyces cerevisiae.
- YACs have been developed in order to clone large sequences of DNA so as to increase the efficiency of the process.
- YACs can clone up to 500 kb of DNA, which is much higher than most traditional cloning vectors.
- Even though these are frequently used as cloning vectors, they are also helpful in other genetic processes like DNA sequencing and analysis.
- These are also unique in their ability to clone the complete sequences of larger genomes that exceed the limits of traditional techniques.
- Since yeast cells are eukaryotic cells, YACs can be used for unstable sequences when cloned in prokaryotic systems.
- These consist of a mixture of functional units from different organisms, but once the insert DNA is cloned, these can function as normally replicating yeast chromosomes.
- There are some limitations with using YAC as vectors as these introduce a high degree of chimerism and insert rearrangement.
- Since these are eukaryotic cells, these are difficult to handle and have lower efficiencies as compared to bacterial artificial chromosomes.
- Different yeast artificial chromosomes have been created over the years that are then used for different purposes.
- One of the most commonly used examples of yeast artificial chromosomes includes pYAC4, which has been extensively used as a cloning vector.
f. Human artificial chromosome
- Human artificial chromosomes are extrachromosomal DNA fragments that act as a new chromosome within the human cell.
- The use of human artificial chromosomes has increased with advances in genetic engineering as it helps overcome problems commonly associated with traditional vector systems.
- HACs can exist as single copy episomes without integration into the host chromosomes allowing long-term stable maintenance.
- Besides, there is no upper limit in the size of the DNA insert to be incorporated into a HAC as entire genomic units can be used to mimic the natural gene expression.
- In spite of numerous advantages, HACs have only been used for studies related to the structure and function of human kinetochores.
- Limitations associated with HACs are due to technical difficulties during gene loading and ill-defined structures of the vectors.
2. Viral vectors
- Viral vectors are one of the most effective means of gene transfer to modify host cells or tissues and manipulate them to express different types of genes.
- The concept of using viruses as vectors arose from the fact that viruses are very effective in transducing their own genetic information into the host cell.
- During viral transduction, the non-essential viral genes are replaced with foreign DNA sequences of therapeutic interest in order to produce recombinant viral vectors.
- Currently, different groups of viruses have been studied doe their possible use as viral vectors to deliver genes to provide transient or permanent transgene expression.
- The use of viral vectors also enables location specificity with unique injection technology within a specific time period.
- Some of the common virus groups considered for viral vectors are adenoviruses, retroviruses, poxviruses, and adeno-associated viruses.
- The choice of a particular virus as a vector depends on a number of factors that include efficiency of transgenic expression, ease of production, safety, and stability.
- Different clinical trials have been held with different potential viral vectors that are suitable for different purposes.
- Adenoviruses have been used for the transfer of tumor suppressor genes in cancer treatment, and retroviruses are studied for their potential use in tissue repair and engineering.
3. Expression vector
- Expression vectors are vectors that enable the expression of cloned genes in order to determine the successful cloning process.
- Usually, cloning vectors do not allow the expression of a cloned gene which is why the use of expression vectors is required.
- The use of expression vectors facilitates the processing of introns in prokaryotes as these are designed with restriction sites next to the regulatory region.
- The restriction sites on the vectors result in splicing of the cloned gene to permit the expression of the gene under the regulatory system.
- The regulatory system in expression vectors consists of a promoter sequence, a termination sequence, along a transcription termination sequence.
- The use of expression vectors is essential to determine the success of a cloning procedure and the efficiency of selective markers on the vectors.
- Expression vectors can be plasmid-based or viral-based that are introduced into the host cells in order to code for particular mRNAs.
- The expression vectors are often used for the production of proteins that can then be visualized by different methods depending on the complexity of the host cell.
- Expression vectors are of varying degrees of complexity depending on whether they are to be used in prokaryotic or eukaryotic cells.
4. Shuttle vector
- Shuttle vectors are that carry origins of replication from two different hosts, which enables them to ‘shuttle’ between the two hosts.
- These vectors contain DNA plasmids that can usually replicate in both mammalian cells as well as bacterial cells.
- Shuttle vectors function as hybrid vectors containing DNA sequences from bacterial plasmids and mammalian viruses.
- The vectors contain three functional DNA sequences involved in the cloning process; a viral replication origin, a bacterial replication origin, and a drug resistance gene.
- The presence of different replication sites and repair sequences enable the recovery and maintenance of these vectors in bacterial cells.
- There are three different shuttle vectors depending on the type of replication system utilized by the vectors.
- Transiently replicating shuttle vectors that need to be recognized by large T antigen in order to replicate in human cells.
- Episomal shuttle vectors work to establish cell lines that can replicate permanently in the form of plasmid DNA containing the DNA insert.
- Integrated shuttle vector undergoes replication only after fusion with particular cell types for gene expression.
5. Secretion vector
- Secretion vectors are a type of specialized expression vector that expresses the cloned genes in order to produce proteins at locations other than the cytoplasm.
- The transport of protein product from the cell is achieved by the fusion of the inset DNA with a nucleotide sequence encoding the peptide of an easily secreted protein.
- The use of secretion vector has many advantages like higher yield, simple purification process, and improved protein stability.
- Secretion vectors can be designed for more than one type of prokaryotes or eukaryotes, including mammals.
- A commonly associated problem with the incorporation of a protein of eukaryotic origin into a prokaryotic host is the overexpression of the protein. This problem is solved by the use of secretion vectors that alleviate the formation of inclusion bodies.
- Secretion vectors have replaced cloning vectors in processes focusing on the production of proteins and the expression of eukaryotic DNA fragments.
Examples of Vectors
The following are some of the examples of vectors that are commonly used for different genetic engineering processes;
pBR322
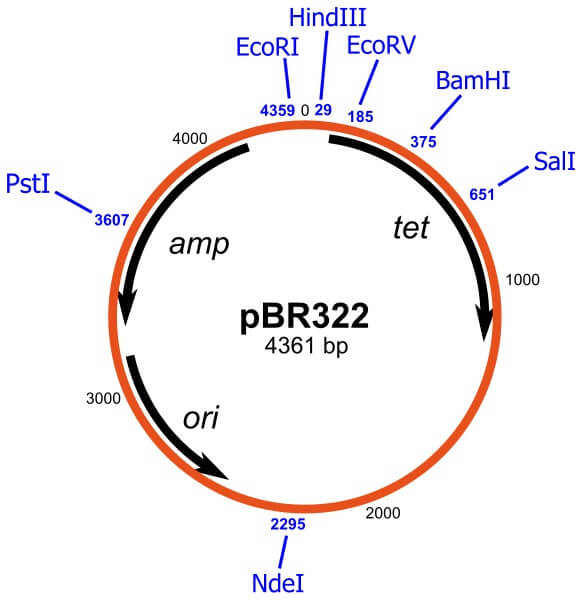
Image Source: Ayacop (+ Yikrazuul).
- pBR322 is a commonly used plasmid cloning vector used in prokaryotes, primarily E. coli.
- The vector consists of an origin of replication from a ColE1-like plasmid, pMB1, an ApR gene (Ampicillin resistance gene) from the transposon, Tn3, and a TcR gene from pSC101.
- pBR322 was designed to overcome the limitations with pBR312 and pBR313, both of which have extraneous DNA sequences and restriction enzyme cleavage sites that affected their function as vectors.
- The structure of pBR322 was designed to maximize the number of restriction enzyme cleavage sites on the vector and to minimize its size.
- The vector contains twenty-one unique restriction enzyme cleavage sites, eleven of which are present in the TcR and ApR genes.
- The structure also facilitates a unique EcoRI cleavage site within the plasmid in order to increase the efficiency of the vector.
- The pBR322 family of vectors was initially created for general cloning purposes in E. coli and other similar prokaryotes; however, over the years, derivatives of the vector have been designed for cloning purposes that specific to a particular organism or a particular function.
- Even though pBR322 has been used for decades as an effective multipurpose cloning vector, it has some limitations.
- The vector might be lost in continuous culture in the absence of selective pressure, which might be a problem in large-scale fermentation of recombinant bacteria.
pUC19
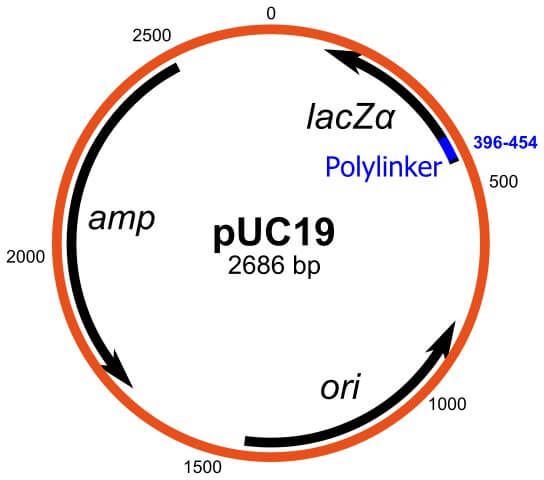
Image Source: Yikrazuul.
- pUC19 is also an example of a plasmid cloning vector that is used for the transfer of recombinant DNA fragments into a host cell.
- The name ‘pUC19’ is given o the vector where the ‘p’ indicates plasmid and ‘UC’ indicates the University of California’ where the vector was designed and constructed.
- The vector has been extensively used for cloning purposes where the host cells containing the plasmid are distinguished from the ones that do not have it by the differences in the color of the colonies on the growth medium.
- The vector is a double-stranded DNA molecule with 2686 bp and a high copy number.
- pUC19 consists of a 54 base-pair cloning site polylinker that further contains 13 different hexanucleotide-specific restriction endonucleases.
- The colony screening after cloning with pUC19 is due to the presence of a selective marker that encodes for the N-terminal fragment of β-galactosidase.
λ phage
- λ-phage is an example of a bacteriophage that infects the bacterial species, Escherichia coli (E. coli).
- This vector is more effective than other plasmid vectors as it has a higher efficiency in entering bacterial cells so as to incorporate the recombinant DNA within the host genome.
- It is a double-stranded DNA bacteriophage that contains an ori sequence requires for replication and a number of DNA sequences encoding regulatory and replicative proteins.
- The phage DNA replicates by the combination of theta and rolling circle replication process to produce a linear dsDNA. It is then followed by the cos sequence, which enables the circularization of the genome after infection.
- The DNA sequences between the two arms of the vector are not essential which are then replaced with the recombinant DNA during cloning.
Applications of Vectors
The application of vectors in molecular biology and genetic engineering has increased with time due to the simplicity, cost-effectiveness, and rapidity of the process. The following are some of the major applications of vectors in molecular biology;
- Cloning vectors are the most important group of vectors that are used for the transfer of foreign DNA into host cells for different purposes.
- One of the most important applications of vectors is to generate engineered organisms for a particular function, like engineering E. coli bacteria for insulin production.
- Vectors can be used to isolate a particular gene sequence within a genome and to determine its nucleotide sequence through DNA sequencing.
- It also helps determine control sequences and regulatory sequences in genomes for their study and analysis.
- Cloning vectors can be used for studying the structure, function, and production of protein in different organisms.
- Phage therapy is a form of therapy that uses bacteriophage vectors to treat different bacterial infections in humans and other animals.
- Vectors can also be used to identify mutations in different regions of DNA sequences as well as to diagnose gene defects related to certain diseases.
- Recombinant DNA technology has been used in clinical microbiology in different approaches like recombinant antigens, recombinant vaccines, and diagnostic probes.
- Recombinant antigens prepared by cloning techniques by using cloning vectors have been used for the screening of diseases like HIV, HCV, and CMV.
- Vectors are one of the components in molecular biology which enable numerous studies related to cell structure, nucleic acid composition, and genetic engineering techniques.
Limitations of Vectors
The following are some of the limitations of vectors;
- Vectors are not very stable due to changes in metabolic energy and changing pH and temperature in different hosts. The stability of vectors depends largely on the type of vector and host genotypes.
- Overexpression of a particular type of genes in the host cell is a common problem associated with the use of vectors.
- The use of a single type of vector might not be sufficient for a particular purpose. The use of multiple vectors is complex and results in difficulties during the process.
- Even though a large number of studies are done in the field of molecular biology for the production of more efficient vectors, it is a time-consuming and expensive process.
References
- Asami, Junko et al. “Bacterial artificial chromosomes as analytical basis for gene transcriptional machineries.” Transgenic research vol. 20,4 (2011): 913-24. doi:10.1007/s11248-010-9469-3
- Basu J, Willard HF. Human artificial chromosomes: potential applications and clinical considerations. Pediatr Clin North Am. 2006 Oct;53(5):843-53, viii. doi: 10.1016/j.pcl.2006.08.013. PMID: 17027613.
- Ramsay M. Yeast artificial chromosome cloning. Mol Biotechnol. 1994 Apr;1(2):181-201. doi: 10.1007/BF02921558. PMID: 7859160.
- Anand R. Yeast artificial chromosomes (YACs) and the analysis of complex genomes. Trends Biotechnol. 1992 Jan-Feb;10(1-2):35-40. doi: 10.1016/0167-7799(92)90165-r. PMID: 1367930.
- Collins J, Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242-6. doi: 10.1073/pnas.75.9.4242. PMID: 360212; PMCID: PMC336088.
- Dhingra G, Kumari R, Bala S, Majumdar S, Malhotra S, Sharma P, Lal S, Cullum J, Lal R. Development of cloning vectors and transformation methods for Amycolatopsis. J Ind Microbiol Biotechnol. 2003 Apr;30(4):195-204. doi: 10.1007/s10295-003-0040-6. Epub 2003 Apr 8. PMID: 12687493.
- Rohweder B, Semmelmann F, Endres C, Sterner R. Standardized cloning vectors for protein production and generation of large gene libraries in Escherichia coli. Biotechniques. 2018 Jan 1;64(1):24-26. doi: 10.2144/000114628. PMID: 29384074.
- van Embden J. (1983) The Use of Cosmids as Cloning Vehicles. In: Walker J.M., Gaastra W. (eds) Techniques in Molecular Biology. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-6563-1_17
- Kazuki, Yasuhiro, and Mitsuo Oshimura. “Human artificial chromosomes for gene delivery and the development of animal models.” Molecular therapy : the journal of the American Society of Gene Therapy vol. 19,9 (2011): 1591-601. doi:10.1038/mt.2011.136
- Kouprina, Natalay et al. “A new generation of human artificial chromosomes for functional genomics and gene therapy.” Cellular and molecular life sciences : CMLS vol. 70,7 (2013): 1135-48. doi:10.1007/s00018-012-1113-3
- Kim, Jung-Hyun et al. “Human artificial chromosome (HAC) vector with a conditional centromere for correction of genetic deficiencies in human cells.” Proceedings of the National Academy of Sciences of the United States of America vol. 108,50 (2011): 20048-53. doi:10.1073/pnas.1114483108
- Rech, E L et al. “Introduction of a yeast artificial chromosome vector into Saccharomyces cerevisiae cells by electroporation.” Nucleic acids research vol. 18,5 (1990): 1313. doi:10.1093/nar/18.5.1313
- Kim, U J et al. “A bacterial artificial chromosome-based framework contig map of human chromosome 22q.” Proceedings of the National Academy of Sciences of the United States of America vol. 93,13 (1996): 6297-301. doi:10.1073/pnas.93.13.6297
- Yamaguchi, Shigeyuki et al. “Application of a bacterial artificial chromosome modification system for a human artificial chromosome vector.” Yonago acta medica vol. 54,1 (2011): 21-31.
- Griffiths AJF, Gelbart WM, Miller JH, et al. Modern Genetic Analysis. New York: W. H. Freeman; 1999. Cloning a Specific Gene. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21450/
- Nora, Luísa Czamanski et al. “The art of vector engineering: towards the construction of next-generation genetic tools.” Microbial biotechnology vol. 12,1 (2019): 125-147. doi:10.1111/1751-7915.13318
- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 7.1, DNA Cloning with Plasmid Vectors. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21498/
- Wang, Yao et al. “Viral vectors as a novel tool for clinical and neuropsychiatric research applications.” General psychiatry vol. 31,2 e000015. 25 Oct. 2018, doi:10.1136/gpsych-2018-000015
- Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol. 2011;737:1-25. doi: 10.1007/978-1-61779-095-9_1. PMID: 21590391.
- Sarasin A. Shuttle vectors for studying mutagenesis in mammalian cells. J Photochem Photobiol B. 1989 Apr;3(2):143-55. doi: 10.1016/1011-1344(89)80057-0. PMID: 2542504.
- Larson, J L, and C L Hershberger. “Shuttle vectors for cloning recombinant DNA in Escherichia coli and Streptomyces griseofuscus C581.” Journal of bacteriology vol. 157,1 (1984): 314-7. doi:10.1128/JB.157.1.314-317.1984
- Balbás P, Soberón X, Merino E, Zurita M, Lomeli H, Valle F, Flores N, Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives–a review. Gene. 1986;50(1-3):3-40. doi: 10.1016/0378-1119(86)90307-0. PMID: 3034735.
- Balbas P, Soberon X, Bolivar F, Rodriguez RL. The plasmid, pBR322. Biotechnology. 1988;10:5-41. doi: 10.1016/b978-0-409-90042-2.50007-6. PMID: 3061523.

THANK U VERY MUCH, IT WAS AMAZING.
so much information and so well condensated.
you have save my life!!!!
Ajoyib malumotlar zo‘r👍
Thank You for such a comprehensive notes on vectors.
Mundhe, R. (2023) What is vector in biology? – plasmid, Cosmid, and Phagemid | biologyideas.com, Biology Ideas. Available at: https://biologyideas.com/vector-in-biology/ (Accessed: April 2, 2023).
This is so amazing!!!!!! What a huge effort. I will pray for whom did this for the rest of my life. Many thanks for this well organized information.
Your notes are really very helpful .
Thanks for the information.
Hi Komal,
Thank you so much for liking our website.