Interesting Science Videos
Bacteriophage Definition
Bacteriophage or Phage is a virus that infects and replicates only within the body of bacteria.
- Bacteriophages were discovered independently by Frederick W. Twort in the U.K and Félix d’Hérelle in France.
- The term ‘bacteriophage’ has been derived from two words; ‘bacteria’ and ‘phagein’, meaning devour. The term was coined by Félix d’Hérelle.
- These are found throughout the world in different environments and are even recognized as one of the most abundant biological agents on earth. These are the most abundant biological particles in water and the second most abundant component of the biomass on land following prokaryotes.
- Bacteriophages that infect bacteria can also infect the members of the domain Archaea.
- Bacteriophages are diverse in their shape size and genome organization depending on the type of bacteria they infect, but the basic composition remains the same.
- All bacteriophages consist of a nucleic acid genome which is enclosed inside a shell of phage-encoded capsid proteins.
- The head structure of different phages might differ, the sizes of phages range between 24-200 nm in length.
- The shape, size, and structure of different bacteriophages are different depending on the type of bacteriophages.
- The studies on bacteriophages have increased over the years, as the scope of their applications has increased.
- The ability of phages to infect and possibly kill infectious bacterial agents puts forward their potential as a possible supplement or replacement for antibiotic agents.
- The mechanism of infection of bacteriophages remains almost the same where they first attach to the host cell and enter their genome into the host cell to suspend the host cellular machinery.
Structure of Bacteriophage
Even though there are different types of phages depending on the type and group of bacteria, they infect, however, all phages share some common characteristics or properties. Some of such characteristics or properties of bacteriophages are:
- Like all other viruses, bacteriophages are also highly species-specific towards their host cell. The bacteriophages only infect a single species of bacteria or even specific strains of bacteria within a species.
- The basic structure of all bacteriophages is the same. They consist of a core of nuclear material surrounded by a protein capsid.
- Bacteriophages exist in three basic structural forms; an icosahedral head with a tail, an icosahedral head without a tail, and a filamentous form.
- The genetic material or nuclear material of bacteriophages can be either DNA or RNA, both of which can either be double-stranded or single-stranded.
- Bacteriophages are obligate intracellular parasites that remain latent outside the host cell and require host cellular machinery to conduct their metabolic activities.
- Like bacteria, bacteriophages are also classified into different orders and families depending on their morphology and genetic material. Some of the commonly studied families include Inoviridae, Tectiviridae, Microviridae, and Rudiviridae.
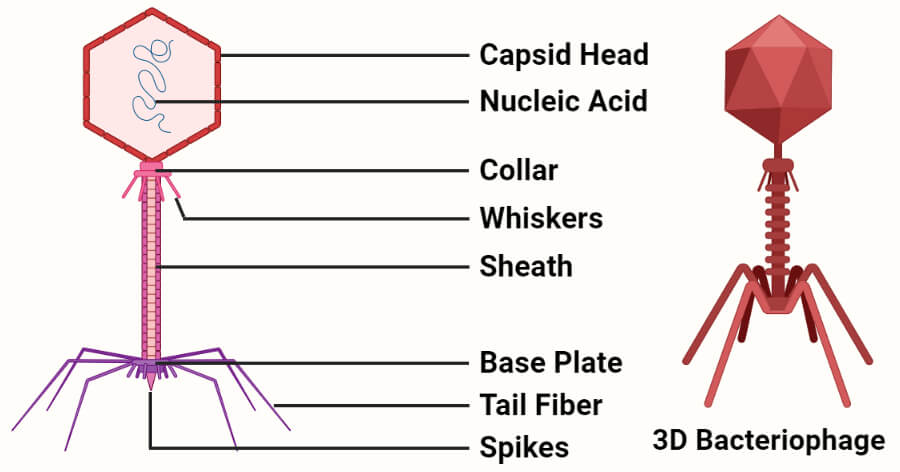
Figure: Structure of Bacteriophage. Created with BioRender.com.
Bacteriophage Models or Types
1. λ phage
- Lambda phage or coliphage λ is a bacteriophage that infects the bacteria belonging to the members of the bacterial species Escherichia coli (E. coli).
- The lambda phage was originally discovered by Esther Lederberg in 1951 in the US during her studies on E. coli under ultraviolet irradiation.
- It belongs to the Siphoviridae family of the order Caudovirales which is defined by the lack of envelope, non-contractile tail, and a linear double-stranded DNA molecule.
- Lambda viruses have been studied for various purposes to understand the lytic and lysogenic lifestyles of various viruses and also as model viruses for viral studies.
- The virus has a temperate life cycle that enables it to either enter into the lytic phase or reside within the host’s genome via lysogeny.
- The structure of the phage particle consists of a protein head or capsid, a non-contractile tail, and tail fibers. The viral genome is present inside the capsid of the virus.
- The non-contractile tail of the virus indicates that the virus cannot force into the cell membrane of the bacteria and must depend on existing pathways to invade the host cell.
- The virus consists of 12-14 different types of proteins comprised of more than 1000 protein molecules and a single DNA molecule present in the phage head.
2. T4 phage
- The T4 virus is a bacteriophage that infects the members of the bacterial species Escherichia coli and thus, is also known as Escherichia virus T4.
- The virus is one of the seven Escherichia coliphages (name T1-T7), which were discovered by Delbruck and coworkers in 1944 as models to study different mechanisms of the phage community.
- The bacteriophage T4 belongs to the Caudovirales order of the Myoviridae family of bacteriophages based on the presence of a non-enveloped head and contractile tail.
- The structure of bacteriophage T4 consists of a protein capsid, called, head which consists of a linear double-stranded DNA molecule.
- At the end of the tail is a 925 Å long and 520 Å diameter contractile tail attached to a special portal at the base of the head.
- There are six short tail fibers emerging from the baseplate that can recognize receptor molecules on the host surface.
- Bacteriophage T-even viruses are among the most commonly studied and researched group of bacteriophages that also are similar to one another in various factors.
- These are also one of the largest and most complicated groups of bacterial viruses as their genetic makeup is made up of about 300 different genes.
Life Cycles of Bacteriophage
Viruses enter the host cell to reproduce during which the virus results in different forms of infections to the host cell. The overall process of the entry of the virus, its replication, and exit from the host cell comprises the lifecycle of viruses. Bacteriophages, like all other viruses, follow a similar trajectory where the virus enters the bacterial host cell in order to replicate. There are two types of lifecycles that differ in the mechanism of DNA replication where, in one, the viral DNA is incorporated into the host DNA, but in the other, the DNA replicates separately from the host DNA. These lifecycles might occur independently or alternatively in different types of bacteriophages.
1. Lytic Cycle
- The lytic cycle is one of the two lifecycles of bacteriophages where the viral DNA remains as a free-floating molecule and replicates separately from the bacterial DNA.
- The lytic cycle usually occurs in virulent phages as the phages result in the destruction of the infected cell membrane during the release of the viral particles.
- The lytic cycle is a virulent infection as it results in the destruction of a cell.
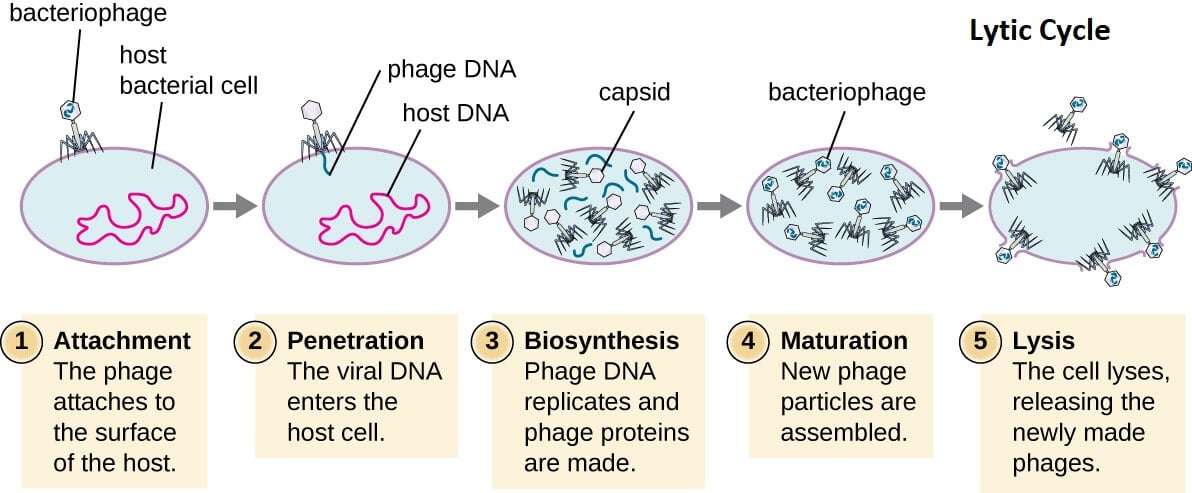
Figure: Lytic Cycle of Bacteriophage. Image Source: Openstax Microbiology.
The lytic lifecycle of bacteriophage is completed in the following steps;
a. Attachment and Penetration
- The first step in the lifecycle of a bacteriophage is attachment, where the ligands on specific molecules on the surface of the viral particles bind to the receptor molecules on the plasma membrane of the host cell.
- The receptors depend on the type of viruses as most orthomyxoviruses use receptors like terminal sialic acid on an oligosaccharide side chain of a cellular glycoprotein.
- The ligand, however, is an aperture at the distal end of each monomer of the trimeric viral hemagglutinin glycoprotein.
- Even though there is a high degree of specificity between the receptors and the ligands, a number of viruses might use the same receptors.
- Besides, some bacteriophages might use other membrane glycoproteins as their receptors.
- Once attached, the virus injects its nuclear material into the cytoplasm of the bacterial cell.
- The viral genome (either DNA or RNA) remains in the cytoplasm, and in some cases becomes circular and resemble the bacterial plasmid.
b. Biosynthesis and Transcription
- Once in the cytoplasm, the viral genome hijacks the host cellular mechanism and utilizes it to produce more viruses.
- In the case of DNA viruses, the DNA undergoes transcription to produce messenger RNA that then directs the ribosome of the host cell.
- In the case of the lytic cycle, the mRNA encodes for various polypeptides, the first of which destroy the host’s DNA.
- In the case of RNA viruses, an enzyme called reverse transcriptase is involved which transcribes the viral RNA into DNA.
- The DNA is then transcribed back to mRNA, which then directs the destruction of host DNA.
- The viral DNA then takes control of the host cell and produces different proteins required for the assembly of new viruses.
- The viral DNA also undergoes replication to produce more genetic material for new viral particles.
- The process of biosynthesis and DNA replication is mediated by different genes and enzymes.
c. Assembly and Lysis
- As biosynthesis and replication continue, a large number of viral proteins and genomes are formed.
- Once enough viral particles are formed and matured, these particles under assembly during which the genetic material of the virus is incorporated into the viral protein, capsid.
- The newly assembled bacteriophages release the enzyme, lysin, into the cytoplasm. The enzyme causes the lysis of the bacterial cell wall, resulting in the release of newly formed phage particles.
- Thus, at the end of the lytic lifecycle, the infected bacterial cell and cell membrane are destroyed.
2. Lysogenic Cycle
- Lysogenic is one of the two lifecycles of bacteriophages defined by the incorporation of the bacteriophage genome into the host genome.
- During the lysogenic lifecycle, the host bacteria continue to live and reproduce normally after the replication of bacteriophages.
- The genetic material of bacteriophage incorporated in the bacterial DNA during the lysogenic lifecycle is called a prophage which can be transmitted to daughter cells during the bacterial cell division.
- The lysogenic cycle is a temperate and non-virulent infection as the bacteriophage doesn’t kill the host cell.
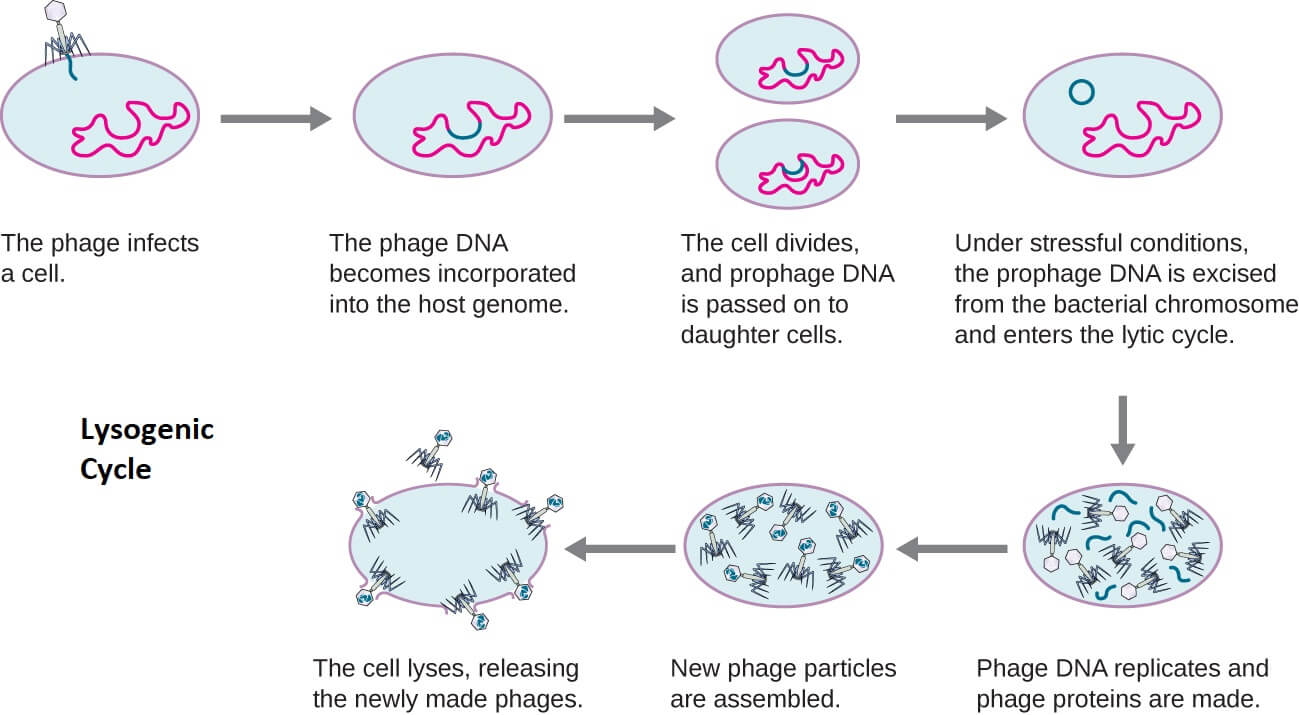
Figure: Lysogenic Cycle of Bacteriophage. Image Source: Openstax Microbiology.
The process of lysogenic lifecycle occurs in the following steps;
a. Attachment and Penetration
- The first step of the lysogenic lifecycle is identical to the first step of the lytic lifecycle.
- The bacteriophage ligands attach to the receptors on the surface of the bacterial cell wall.
- The attachment is highly specific as it is determined by the interaction between the ligands and the receptors present on the surface of the bacterial cell wall.
- After attachment, the viral genome is injected into the cytoplasm of the host cell.
- The infective viral DNA or prophage is then incorporated into the host chromosome, which converts the infective prophage into a non-infective prophage.
b. Replication
- The viral DNA then uses the host machinery to replicate as it continues to replicate with the host chromosomes during cell division.
- In some cases, the prophage might be ejected from the host chromosome, and the viral DNA might enter the lytic cycle.
- Unlike the lytic cycle, the bacterial cellular mechanism is not hijacked by the viral particles, and no biosynthesis of viral proteins takes place.
- The prophage, however, can be transferred to the daughter cells during the bacterial cell division.
- The process of replication continues until there are some stressors which can either be physical stressors like UV radiation, low nutrient condition or chemical, which might result in the transition of the lysogenic cycle into the lytic cycle.
- Once converted into the lytic cycle, the viral DNA undergoes transcription to produce viral proteins. The proteins and viral genome are then assembled to form complete viral particles which then are released from the host cell by lysis.
Lytic Cycle vs Lysogenic Cycle (14 major differences)
| Characteristics | Lytic Cycle | Lysogenic Cycle |
| Definition | The lytic cycle is a type of lifecycle of bacteriophages where the viral DNA remains as a free-floating molecule and replicates separately from the bacterial DNA. | Lysogenic is another type of lifecycle of bacteriophages which is defined by the incorporation of the bacteriophage genome into the host genome. |
| Also called | The lytic cycle is also called the infective cycle or virulent cycle. | The lysogenic cycle is also called a temperate cycle or non-virulent cycle. |
| Viral DNA | In the lytic cycle, the viral DNA remains in the cytoplasm of the host cell. | In the lysogenic cycle, the viral DNA is incorporated into the host chromosome. |
| Prophage | No prophage is present in the lytic cycle. | The lysogenic cycle consists of a prophage stage. |
| Host DNA | Host DNA is destroyed by various proteins encoded by the viral DNA. | The host DNA is not affected by the viral DNA. |
| Viral replication | The viral DNA replication occurs separately from the host DNA replication. | Viral DNA replication occurs along with the host DNA replication. |
| The productivity of viral DNA | The productivity of viral DNA and viral proteins is high. | The productivity of viral DNA and viral proteins is low. |
| Host cellular mechanism | Host cellular mechanism is completely hijacked by the viral DNA. | Host cellular mechanisms remain unaffected. |
| Duration | The lytic cycle is immediate and is completed within a short period of time. | The lysogenic cycle takes a longer period of time. |
| Transition | The lytic cycle cannot transition into a lysogenic cycle. | The lysogenic cycle can transition into the lytic cycle. |
| Infection | As the cycle is an infective cycle, symptoms of viral infections can be observed. | The cycle is a non-infective cycle that doesn’t result in symptoms. |
| Transfer | The viral DNA cannot be transferred from the host cell to the daughter cell during the lytic cycle. | The viral DNA can be transferred into the daughter cell during the lysogenic cycle. |
| Genetic recombination | The lytic cycle doesn’t allow genetic recombination of the host chromosome. | The lysogenic cycle allows the genetic recombination of the host chromosome. |
| Lysis of host cell | The lytic cycle ends with the lysis of the host cell. | The lysogenic cycle doesn’t result in the lysis of the host cell. |
Applications of Bacteriophages
Bacteriophages have been considered to be potential antibacterial therapeutics for the treatment of various infectious bacterial diseases in humans and animals. In the beginning, the clinical application of bacteriophages was limited to the treatment of acute intestinal infections and skin infections. Later, however, the application of bacteriophages in surgical practices for the treatment of prurient infectious complications was initiated. The following are some of the application of bacteriophages in different areas;
Treatment of bacterial infections
- With the increasing cases of bacterial resistance against numerous antibiotics, the potential use of bacteriophage a possible treatment has been explored.
- As the bacteriophage infects only bacteria and is harmless to humans, the administration of such bacteriophages into humans helps in the destruction of such infectious bacteria.
- Besides, the application of bacteriophages on burn wounds has shown to reduce the chances of infection and sepsis by a large number.
In food hygiene and safety
- Bacteriophages are used to control and eliminate bacterial contaminants from food surfaces and food-borne spoilage.
- Bacteriophages are highly specific, which makes them attractive for sanitization of ready-to-eat foods like milk, vegetables, and meat products.
- Many bacteriophages have been commercialized for their use as spray sanitizers to disinfect cattle hides prior to slaughter in order to reduce contamination in the meat.
- Some bacteriophages are also useful as surface and environment decontaminants as they can disinfect stainless stain as efficiently as a quaternary ammonium compound.
In agriculture
- Some bacteriophages that are specific to plant bacteria have also found their application in agriculture.
- These phages are used for the treatment and prevention of bacterial diseases in plants. The use of bacteriophages in the place of antibiotics prevent the clumping of antibiotics on the plant surface, which then might be harmful to the health of the consumers.
What is Phage Therapy?
Phage therapy or viral therapy is the use of bacteriophages to treat various bacterial infections.
- Even though the concept of using bacterial viruses to treat bacterial infections is only recently considered as an alternative to antibiotics, this method has a contentious history in western medicine.
- However, the current knowledge and application of phage therapy have advanced well beyond traditional methods.
- The concept of phage therapy actually began with the first discovery of bacteriophages by Twort and d’Herelie in 1917.
- Over time, the use of phage therapy has been continued for a range of clinically significant pathogens based on recent investigations using animal models.
- Human trials for phage therapy began almost a century ago, and it is currently used for the treatment of common bacterial pathogens like Staphylococcus aureus, Enterococcus, Proteus, and Pseudomonas aeruginosa.
- The effective applications of phage therapy range from surgical to gastroenterological treatment that can be both therapeutic and prophylactic.
- Even though no phage therapy products are yet approved for clinical use in humans, commercial phage preparations have been used as biocontrol agents in the food industry.
- These preparations are used against common food pathogens like Salmonella, Campylobacter, and Listeria monocytogenes.
- Phage therapy is often compared with antibiotics, and it has been noted that phage therapy has various advantages over antibiotics.
- There are fewer to no side effects of phage therapy, and phages are even effective against the bacterial population present in biofilms.
- In addition to the use of phages against bacterial infections, the use of phage-encoded lytic enzymes is also conducted.
- These enzymes tend to be similar to the antimicrobial eukaryotic enzyme lysozyme that causes lysis of the bacterial cells.
Image Source: Cleveland Clinic.
Phage Therapy Principle
The basic principle of the use of phage therapy as a possible method of treatment and prevention of bacterial infection is the use of bacteriophages to destroy bacterial cells involved in infections. Besides, there are different phage-encoded enzymes that can also be administered to brings about the lysis of the bacterial cells. The principle of phage therapy can be explained in two different ways depending on the use of either phage (active therapy) or phage-encoded enzymes
1. Principle of active phage therapy
- The use of phages as a mode of therapy begins with the administration of phages. The phages reach the bloodstream (from oral dose) within 2-4 hours, and they are found in the internal organs in about 10 hours.
- The bactericidal activity of the phages is the result of the replication of viruses through the lytic cycle within the host cell.
- Studies have revealed that not all phages replicate similarly, and there might be major differences in the lytic and lysogenic cycle of pages.
- The lysis of host bacteria via the lytic cycle is a complex process that is brought about by a cascade of events involving several structural and regulatory genes.
- However, in order to determine the efficiency of the agents, there are different values that are to be understood.
a. Proliferation density threshold
- The growth of the phage population depends on the density of the bacteria.
- The increase in phage population with the increase in bacterial population occurs up to a point which is called a threshold.
- The threshold determines whether the probability of a free phage can meet and infect a susceptible bacterial cell exceeds that of a phage being lost from the system.
- Therefore, the success of active phase therapy depends not only on the type of phage and bacteria involved but also on the density of bacterial at any time.
b. Optimal timing
- Another thing to consider in active phage therapy is the timing of the use of phages so that they are active against the bacterial species.
- The inoculation of phages should be done at a particular time when the bacterial density reaches a particular value that is within the threshold value for the phages.
- This time is called proliferation time, and thus, the inoculation of phages should be done at a point that is close to the proliferation time.
2. Principle of phage-encoded enzymes
- There are two major classes of phage lysin that are involved in the lysis of the bacterial cells.
- The proteins are transmembrane protein holing and peptidoglycan cell wall hydrolase called endolysin. These proteins work together in triggering the lysis of the bacterial cell.
- The holin protein acts as a molecular clock in the lytic cycle. During the viral assembly in the bacterial cell, the enzyme molecules accumulate in the cell membrane.
- As the lytic cycle continues, the protein triggers the opening of the cytoplasmic side of the cell membrane, thus allowing the lysin proteins to access and hydrolyze the cell wall.
- These proteins are fast, potent, and inactive against eukaryotic cells which increases the interest in their use as therapeutic agents.
- It has been demonstrated that the use of phage lysins and antimicrobials is more effective against various bacteria than using antibiotics alone.
Advantages of Phage Therapy
- Bacteria that are infected by obligately lytic phages are incapable of regaining their viability.
- The phage population can increase in response to the increase of bacterial density up to a point. This process is called auto-dosing.
- Bacteriophages are inherently nontoxic as they are made up of nucleic acids and proteins. However, in some cases, the viruses can interact with the immune system; thus, most phage therapies use highly purified phage preparations.
- As a result of their high host specificity, bacteriophages only infect specific strains of bacteria. This results in minimal to no disruption of the normal flora.
- The narrow host range of most phages limits the number of bacterial types which can result in phage-resistance mechanisms.
- The mechanism of bacterial lysis by phages is completely different from that of antibiotics, which allows the use of phages against antibiotic-resistant infections.
- New phages that are active against many pathogenic bacteria can be easily discovered from sewage and other waste materials.
- Phages are versatile agents and thus, can be used in combination with antibiotics and can also be converted into different forms like liquid, creams, or solids.
- Phages are capable of clearing biofilms of some bacterial phages as a result of their ability to actively penetrate into the biofilms.
Limitations of Phage Therapy
- Not all phages make for good therapeutics, and the use of temperate phages as therapeutic is problematic as it might result in the conversion of phage-sensitive bacteria into insensitive ones and the encoding of bacterial virulence.
- As phages are highly specific and only infect a few strains of bacteria, they have a narrow host range. As a result, different phages are required for different bacterial infections.
- As phages are protein-based live biological agents, there is a possibility of the interaction between the phage and the immune system of the patient.
- Due to the massive diversity of phages, it is difficult to create a phage cocktail when compared to designing a regimen for combination antibiotic therapy.
- Phages are often misinterpreted by the general public as being equivalent to viruses that cause human diseases, which limits their use.
Phage Therapy vs Antibiotics (10 major differences)
| Characteristics | Phage Therapy | Antibiotics |
| Definition | Phage therapy or viral therapy is the use of bacteriophages to treat various bacterial infections. | Antibiotic is an antimicrobial agent that is effective in the treatment of bacterial infections. |
| Safety | Phage therapy is comparatively safer than antibiotics as there are minimal side effects to the patients. | There are well documented adverse reactions to antibiotics that result in neurotoxicity, cardiotoxicity, and hepatotoxicity. |
| Specificity | Phages are highly specific, and thus, a phage can only be used for a few bacterial strains. | Antibiotics have a broad spectrum that can affect more than a single target organism. |
| Resistance | Resistance against phages can occur, but it is usually limited to a single target bacteria. | Resistance against antibiotics is a common phenomenon, and it is not limited to the targeted bacteria. |
| Efficiency against biofilms | Phages are active against biofilms as they can penetrate biofilms to infect the bacterial population present underneath. | Antibiotics are ineffective against biofilms. |
| Development | The development and isolation of phages are comparatively easy as many phages can be obtained from sewage and waste materials with high bacterial density. | The development of antibiotics is a time-consuming and expensive process. |
| Immune system | Some phages might interact with the immune system of the patients and result in undesired effects. | Antibiotics do not interact with the immune system of the patients. |
| Guidelines | There are no guidelines for the use of phages as therapeutic agents. | There are specific guidelines for the use of antibiotics by a different organization. |
| Combination therapy | The development of combination phage therapy is tough. | The development of a combination antibiotic therapy is fairly easy. |
| Administration | The administration of some forms of phage therapy might be difficult. | The administration of antibiotics is quite easy. |
What is Phage typing?
Phage typing is a method of fingerprinting disease-causing agents for epidermal investigation and surveillance.
- Phage typing is a rapid, economical, and reproducible method that doesn’t require specialized tools for the detection of bacteria.
- The method has been used for years to determine the relationship between species and to study outbreaks.
- The principle of phage typing is based on the culture of bacteria under investigation as a lawn inoculum. The culture is then subjected to attack by different phages. Depending on the specificity between the phages and the bacterial strains, phages will lyse the bacterial colony, which can then be visualized or measured by different methods.
- The method of phage typing is essential for the determination of the source of infections, the route of transmission, outbreaks, and epidemics.
- Phage typing is a universal method for the typing of Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella Typhi.
Limitations or Challenges of Bacteriophages
- Bacteriophages are tiny particles that are difficult to study without appropriate microscopes and other equipment.
- The use of bacteriophages is limited due to the perception of bacteriophages as human viruses that might result in viral infections in humans.
- The information of most bacteriophages is limited as a result of the difficulty in the method of isolation and identification of such viruses.
References
- Kasman LM, Porter LD. Bacteriophages. [Updated 2020 Oct 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493185/
- Griffiths AJF, Miller JH, Suzuki DT, et al. An Introduction to Genetic Analysis. 7th edition. New York: W. H. Freeman; 2000. Lambda phage: a complex of operons. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21856/
- Casjens, Sherwood R, and Roger W Hendrix. “Bacteriophage lambda: Early pioneer and still relevant.” Virology vol. 479-480 (2015): 310-30. doi:10.1016/j.virol.2015.02.010
- Yap, Moh Lan, and Michael G Rossmann. “Structure and function of bacteriophage T4.” Future microbiology vol. 9,12 (2014): 1319-27. doi:10.2217/fmb.14.91
- Weigel C, Seitz H. Bacteriophage replication modules. FEMS Microbiol Rev. 2006 May;30(3):321-81. doi: 10.1111/j.1574-6976.2006.00015.x. PMID: 16594962.
- Paul C. M. Fogg, Heather E. Allison, Jon R. Saunders, Alan J. McCarthy. Bacteriophage Lambda: a Paradigm Revisited. Journal of Virology Jun 2010, 84 (13) 6876-6879; DOI: 10.1128/JVI.02177-09
- Howard-Varona, Cristina et al. “Lysogeny in nature: mechanisms, impact and ecology of temperate phages.” The ISME journal vol. 11,7 (2017): 1511-1520. doi:10.1038/ismej.2017.16
- Aminov, Rustam & Caplin, Jonathan & Chanishvili, Nina & Coffey, Aidan & Cooper, Ian & De Vos, Daniel & Doška, Ji & Friman, Ville-Petri & Kurtböke, Dilber & Pantůček, Roman & Pirnay, Jean-Paul & Resch, Gregory & Rohde, Christine & Sybesma, Wilbert & Wittmann, Johannes. (2017). Application of Bacteriophages. Microbiology Australia. 38. 63-66. 10.1071/MA17029.
- Payne RJ, Jansen VA. Pharmacokinetic principles of bacteriophage therapy. Clin Pharmacokinet. 2003;42(4):315-25. doi: 10.2165/00003088-200342040-00002. PMID: 12648024.
- Lin, Derek M et al. “Phage therapy: An alternative to antibiotics in the age of multi-drug resistance.” World journal of gastrointestinal pharmacology and therapeutics vol. 8,3 (2017): 162-173. doi:10.4292/wjgpt.v8.i3.162
- Chirakadze I., Perets A., Ahmed R. (2009) Phage Typing. In: Clokie M.R., Kropinski A.M. (eds) Bacteriophages. Methods in Molecular Biology™, vol 502. Humana Press. https://doi.org/10.1007/978-1-60327-565-1_17
- Loc-Carrillo, Catherine, and Stephen T Abedon. “Pros and cons of phage therapy.” Bacteriophage vol. 1,2 (2011): 111-114. doi:10.4161/bact.1.2.14590
- Liliam K. Harada, Erica C. Silva, Welida F. Campos, Fernando S. Del Fiol, Marta Vila, Krystyna Dąbrowska, Victor N. Krylov, Victor M. Balcão. Biotechnological applications of bacteriophages: State of the art. Microbiological Research. Volumes 212–213. 2018. Pages 38-58. https://doi.org/10.1016/j.micres.2018.04.007.
- Morozova Vera V., Vlassov Valentin V., Tikunova Nina V. Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Frontiers in Microbiology. VOL 9. YEAR 2018. PAGES 1696. DOI=10.3389/fmicb.2018.01696
- Principi Nicola, Silvestri Ettore, Esposito Susanna. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Frontiers in Pharmacology. VOL 10. 2019. 513. DOI=10.3389/fphar.2019.00513

Wow. I’m doing a vigorous research about phages and seeing this work make it easy for me. It’s a nice work maam.
Thank you.
I wants to know more about isolation and characterization of potent phages, characteristics of phage resistant bacteria and how to develop cock tails of bacteriophages.
Thank you for your support.
Sure, we will try to write content on those things. 🙂