- Yeast artificial chromosomes (YACs) are genetically engineered chromosomes derived from the DNA of the yeast.
- It is a human-engineered DNA molecule used to clone DNA sequences in yeast cells.
- They are the products of a recombinant DNA cloning methodology to isolate and propagate very large segments of DNA in a yeast host.
- By inserting large fragments of DNA, the inserted sequences can be cloned and physically mapped using a process called chromosome walking.
- The amount of DNA that can be cloned into a YAC is, on average, from 200 to 500 kb.
- However, as much as 1 Mb (mega, 106) can be cloned into a YAC.
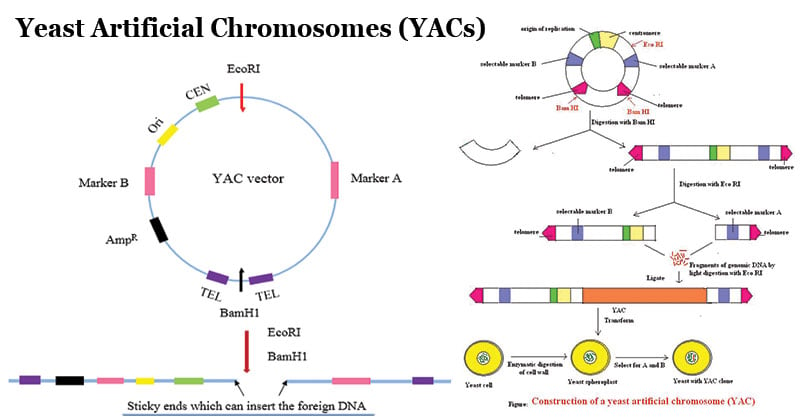
Image Source: https://orbitbiotech.com and https://en.wikibooks.org
Interesting Science Videos
Structure of Yeast Artificial Chromosomes
A yeast artificial chromosome cloning vector consists of two copies of a yeast telomeric sequence (telomeres are the sequences at the ends of chromosomes), a yeast centromere, a yeast ars (an autonomously replicating sequence where DNA replication begins), and appropriate selectable markers.
Working Principle of Yeast Artificial Chromosomes
The yeast artificial chromosome, which is often shortened to YAC, is an artificially constructed system that can undergo replication. The design of a YAC allows extremely large segments of genetic material to be inserted. Subsequent rounds of replication produce many copies of the inserted sequence, in a genetic procedure known as cloning.
- The principle is similar to that for plasmids or cosmids.
- The experimenter introduces some typical elements that are necessary for correct replication.
- In the case of YACs, the replication origins are the centromeres and telomeres of the yeast chromosomes, which must be inserted into the DNA being cloned.
- The constructs can be transformed in yeast Spheroplast and are then replicated there.
- In contrast to the vectors, YACs are not circular; they are made of linear DNA.
Process of Yeast Artificial Chromosomes
- YAC vector is initially propagated as circular plasmid inside bacterial host utilizing bacterial ori sequence.
- The circular plasmid is cut at a specific site using restriction enzymes to generate a linear chromosome with two telomere sites at terminals.
- The linear chromosome is again digested at a specific site with two arms with different selection marker.
- The genomic insert is then ligated into YAC vector using DNA ligase enzyme.
- The recombinant vectors are transformed into yeast cells and screened for the selection markers to obtain recombinant colonies.
Advantages of Yeast Artificial Chromosomes
- Yeast artificial chromosomes (YACs) provide the largest insert capacity of any cloning system.
- Yeast expression vectors, such as YACs, YIPs (yeast integrating plasmids), and YEPs (yeast episomal plasmids), have advantageous over bacterial artificial chromosomes (BACs). They can be used to express eukaryotic proteins that require post-translational modification.
- A major advantage of cloning in yeast, a eukaryote, is that many sequences that are unstable, underrepresented, or absent when cloned into prokaryotic systems, remain stable and intact in YAC clones.
- It is possible to reintroduce YACs intact into mammalian cells where the introduced mammalian genes are expressed and used to study the functions of genes in the context of flanking sequences.
Uses of Yeast Artificial Chromosomes
- Yeast artificial chromosomes (YACs) were originally constructed in order to study chromosome behavior in mitosis and meiosis without the complications of manipulating and destabilizing native chromosomes.
- YACs representing contiguous stretches of genomic DNA (YAC contigs) have provided a physical map framework for the human, mouse, and even Arabidopsis genomes.
- YACs are extremely popular for those trying to analyze entire genomes.
Limitations of Yeast Artificial Chromosomes
- A problem encountered in constructing and using YAC libraries is that they typically contain clones that are chimeric, i.e., contain DNA in a single clone from different locations in the genome.
- YAC clones frequently contain deletions, rearrangements, or noncontiguous pieces of the cloned DNA. As a result, each YAC clone must be carefully analyzed to be sure that no rearrangements of the DNA have occurred.
- The efficiency of cloning is low (about 1000 clones are obtained per microgram of vector and insert DNA).
- YACs have been found to be less stable than BACs.
- The yield of YAC DNA isolated from a yeast clone containing a YAC is quite low.
- The YAC DNA is only a few percents of the total DNA in the recombinant yeast cell. It is difficult to obtain even 1 μg of YAC DNA.
- The cloning of YACs is too complicated to be carried out by a lone researcher.
References
- https://www.sciencedirect.com/topics/neuroscience/yeast-artificial-chromosome
- https://nptel.ac.in/courses/102103013/module1/lec4/11.html
- https://www.ncbi.nlm.nih.gov/pubmed/7859160
- https://www.ncbi.nlm.nih.gov/pubmed/9291964
- https://www.slideshare.net/gurya87/yeast-artificial-chromosomes-yacs-44970900
- https://www.encyclopedia.com/science-and-technology/biology-and-genetics/cell-biology/yeast-artificial-chromosome-yac
- https://www.britannica.com/science/yeast-artificial-chromosome

This’s so amazing!
But how can one reference using your article?
Please upload it for BAC and MAC just like this.
Very good work. please keep going on… students are very appreciating these note.
please do upload all the vectors use in RDT ..