- Deoxycholate Citrate Agar is a modification of Leifson formula and is recommended for the isolation of Salmonella and Shigella spp.
- This medium is similar to deoxycholate agar in comparison but is moderately more selective for enteric pathogens owing to increased concentrations of both citrate and deoxycholate salts.
- Sodium deoxycholate at pH 7.3 to 7.5 is inhibitory for gram-positive bacteria. Citrate salts, in the concentration included in the formulation, are inhibitory to gram-positive bacteria and most other normal intestinal organisms.
- It is thus a selective and differential medium, used commonly for the isolation of enteric pathogens.
Interesting Science Videos
Composition of Deoxycholate Citrate Agar (DCA)
| Ingredients | Gms/liter |
| HI solids | 10.00 |
| Proteose peptone | 10.00 |
| Lactose | 10.00 |
| Sodium deoxycholate | 5.000 |
| Neutral red | 0.020 |
| Sodium citrate | 20.00 |
| Ferric ammonium citrate | 2.000 |
| Agar | 13.50 |
Final pH (at 25°C) 7.5±0.2
Principle of Deoxycholate Citrate Agar (DCA)
- HI solids is a source of carbon and nitrogen and result in the inhibition of coliforms.
- Proteose peptone provides carbon, nitrogen, vitamins and minerals.
- Coliform bacteria and gram-positive bacteria are inhibited or greatly suppressed due to sodium deoxycholate, sodium citrate, and ferric ammonium citrate.
- Dipotassium phosphate buffers the medium.
- Lactose helps in differentiating enteric bacilli, as lactose fermenters produce red colonies while lactose non-fermenters produce colourless colonies.
- Coliform bacteria, if present form pink colonies on this medium.
- The degradation of lactose causes acidification of the medium surrounding the relevant colonies and the pH indicator neutral red changes its color to red. These colonies usually are also surrounded by a turbid zone of precipitated deoxycholic acid due to acidification of the medium.
- Sodium deoxycholate combines with neutral red in an acidic environment, causing the dye to go out of the solution with the subsequent precipitation of deoxycholate.
- The reduction of ferric ammonium citrate to iron sulfide is indicated by the formation of black iron sulfide.
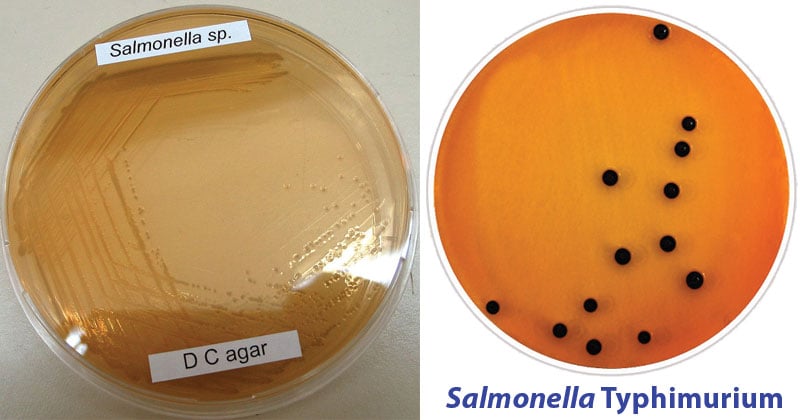
- Salmonella and Shigella species do not ferment lactose but Salmonella may produce H2S, forming colorless colonies with or without black centers.
- Citrate and iron (Fe) combination has a strong hydrolyzing effect on agar when the medium is heated, producing a soft and unelastic agar.
Preparation and Method of Use of Deoxycholate Citrate Agar (DCA)
- Suspend 70.52 grams in 1000 ml of distilled water.
- Heat to boiling to dissolve the medium completely.
Note: DO NOT AUTOCLAVE. Avoid excessive heating as it is detrimental to the medium.
- Cool to 45-50°C. Mix well and pour into sterile Petri plates.
- Dry the agar surface before use.
- Inoculate the medium heavily with feces or rectal swabs, spreading part of the original inoculum in order to obtain well-separated colonies on some portion of the plate.
- Incubate for 18-24 hours at 35°C.
- If organisms are late developers or if no non-lactose fermenters are observed, incubate for a further 24 hours.
Result Interpretation on Deoxycholate Citrate Agar (DCA)
Lactose non-fermenters produce transparent, colorless to light pink or tan-colored colonies with or without black centers.
Lactose fermenters produce a red colony with or without a bile precipitate.
| Organisms | Growth |
| Escherichia coli | Poor growth; pink with bile precipitate negative reaction for H2S |
| Salmonella Enteritidis | Good-luxuriant growth; colorless; positive reaction for H2S, black centered colonies |
| Salmonella Typhimurium | Good-luxuriant growth; colorless; positive reaction for H2S, black centered colonies |
| Shigella flexneri | Good growth; colorless |
| Salmonella Abony | Good-luxuriant growth; colorless; positive reaction for H2S, black centered colonies |
| Shigella sonnei | Colonies are smooth and initially colorless, becoming pale pink on further incubation due to late lactose fermentation |
| Enterobacter/Klebsiella spp. | Large, pale mucoid colonies with the pink center |
Uses of Deoxycholate Citrate Agar (DCA)
- Deoxycholate Citrate Agar is used for the isolation and differentiation of Gram-negative enteric bacilli in a laboratory setting.
- It is particularly useful for the isolation of organisms that cause bacillary dysentery, Salmonella strains that cause food poisoning and Salmonella Paratyphi.
Limitations of Deoxycholate Citrate Agar (DCA)
- Suspected pathogens must be subcultured on a less inhibitory medium prior to identification.
- As there are many bacteria that also look like Salmonella on DCA, it is widely recommended that more selective agars are used for the identification of Salmonella, namely xylose lysine deoxycholate (XLD) agar.
- This growth medium is heat-sensitive and should be poured and cooled as soon as possible after the addition of the deoxycholate, otherwise, it tends to become very soft and difficult to handle.
- For the routine examination of stool and urine specimens, it is suggested that other media such as MacConkey Agar, Bismuth Sulphite Agar etc. be used in conjunction with this medium.
- Further biochemical identification is required for confirmation of species.
- Due to nutritional variations, some organisms may show poor growth.
References
- http://himedialabs.com/TD/M065.pdf
- https://www.usbio.net/culture-media
- https://www.bd.com/europe/regulatory/Assets/IFU/Difco_BBL/227410.pdf
- http://foodsafety.neogen.com/uk/deoxycholate-citrate-agar
- Speck M. (Ed.), 1984, Compendium of Methods for the Microbiological Examination of Foods, 2nd ed., APHA, Washington, D.C
- https://www.thomassci.com/Laboratory-Supplies/Microbiological-Media/_/Deoxycholate-Citrate-Agar-Modified-Hynes
- https://www.sigmaaldrich.com/catalog/product/sial/d7809?lang=en®ion=US
