The complement system constitutes a cascade of interactions between a large number of heat-labile plasma proteins called complements. These proteins are inactively circulating in the plasma and are functional when cleaved into peptide fragments. The activation of the complement system occurs through three different pathways: the classical pathway, the mannose-binding lectin (MBL) pathway, and the alternative pathway.
Interesting Science Videos
What is the classical pathway of the complement system?
The classical pathway of the complement system is activated by the formation of antigen-antibody complexes (immune complexes). Other activators include apoptotic cells, C-reactive protein, bacterial polysaccharides, and nucleic acids, which trigger classical pathways in antibody independent manner. The complement proteins in this pathway are named numerically from 1 to 9 (C1 to C9), each with subunits of their own. All the larger subunits of these proteins are designated as “b” and smaller subunits as “a” while the reverse case occurs in the case of protein C2 (C2a is a larger fragment and C2b is a smaller fragment).
Steps/mechanism/process of classical pathway of the complement system
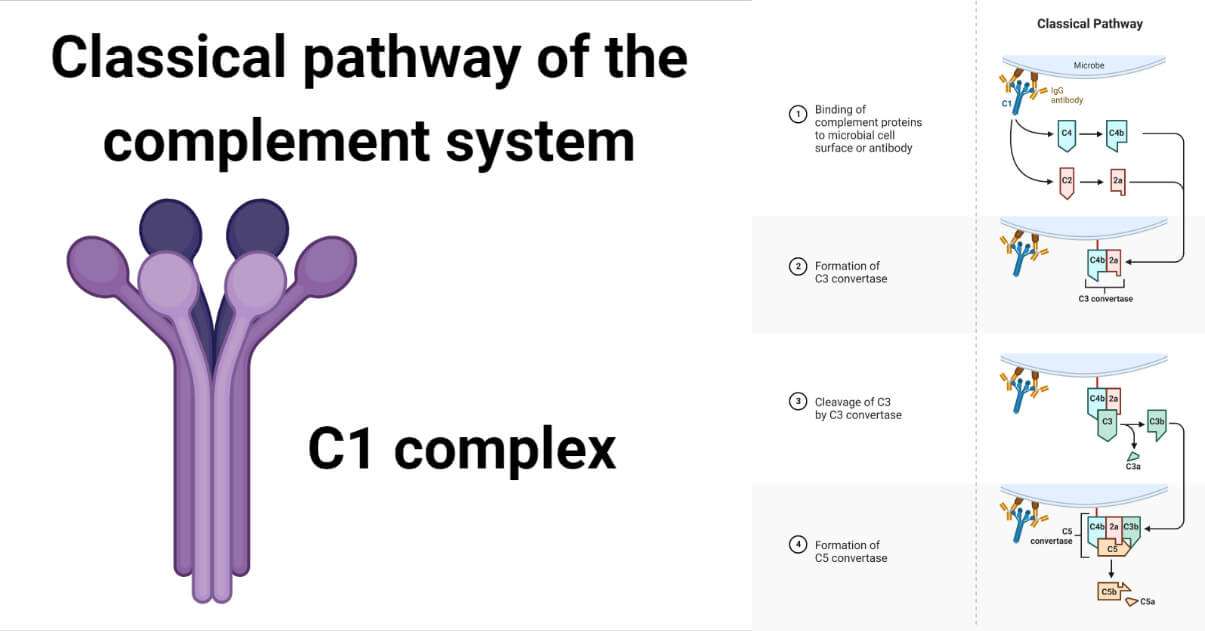
The classical pathway initiates when immune complexes bind to the C1 complex. The pathway continues in the following described way:
- The formation of antigen-antibody complex induces a conformational change in the Fc portion of the antibody, exposing a binding site for the C1 component of complement. C1 protein is a macromolecular complex with the following subunits:
1 C1q, 2 C1r, 2 C1s
C1q has six subunits, each composed of three homologous chains (A, B, and C) with extended tails forming six globular heads. Each globular head acts as a binding site for antibody Fc.
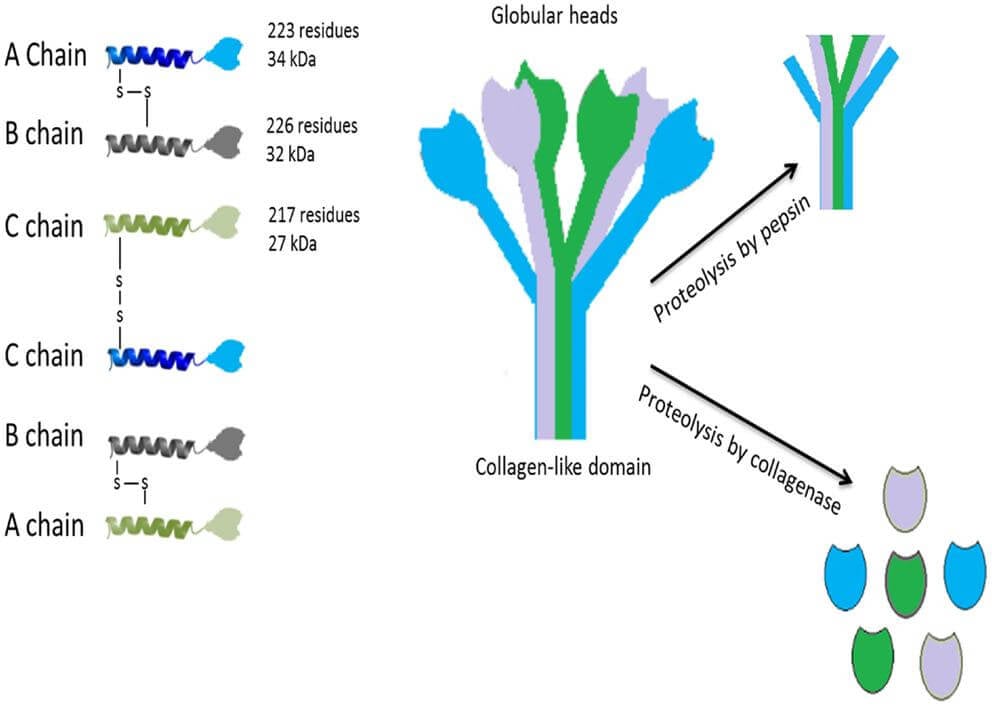
C1q globular heads must bind at least two Fc sites of the antibody to maintain stable C1-antibody interaction. Antigen-bound IgM undergoes conformational change exposing at least three binding sites for C1q whereas IgG has only one binding site for C1q.
- As C1q binds to antibodies through its globular heads, conformational changes occur in the C1r proenzyme. The activated C1r now cleaves C1s activating C1s serine protease.
- Activated C1s tend to activate another component, C4, by cleavage. This bound C1s cleaves C4 forming C4b, which binds covalently to the bacterial surface membrane.
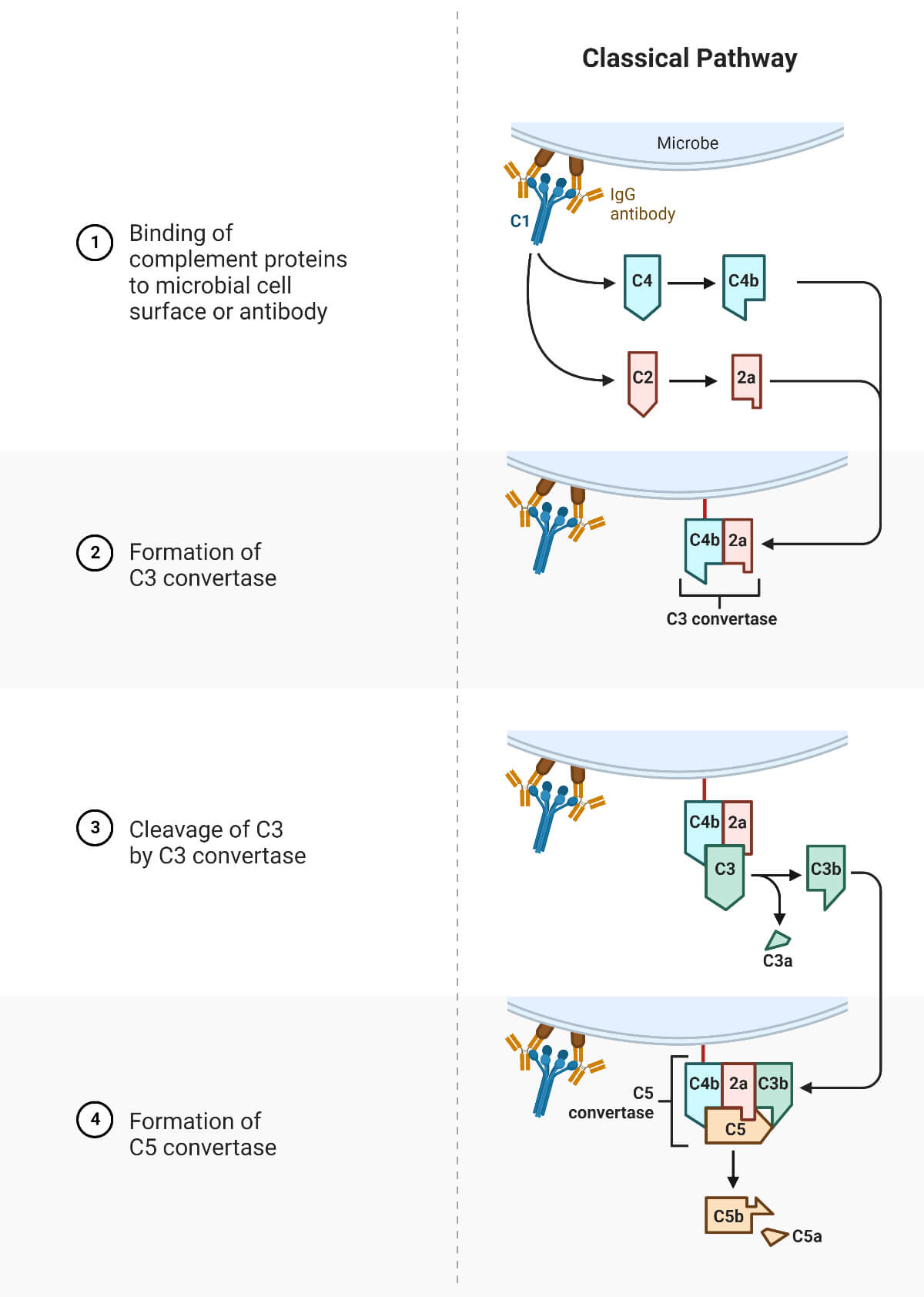
- C4b binds C2, thereby exposing it to cleavage by C1s, forming the C4b2a enzyme complex. This enzyme complex is called C3 convertase and catalyzes the central reaction of the complement pathway. This enzyme deposits a large number of C3b molecules on the surface of the pathogen.
- This membrane-bound enzyme complex C4b2a3b is called C5 convertase. This enzyme in turn, cleaves C5 into C5a and C5b.
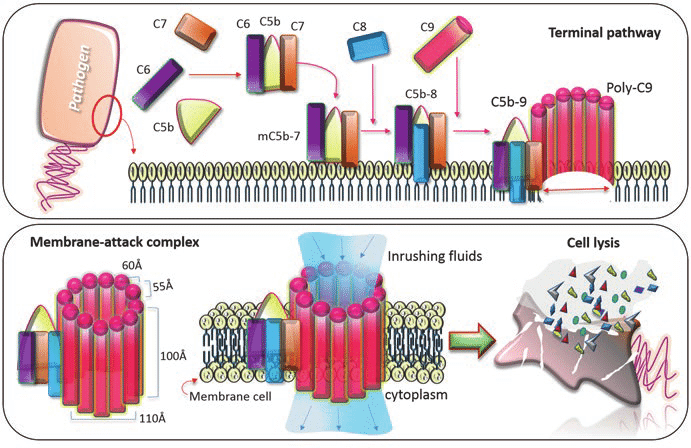
- C5b then triggers the formation of membrane attack complex (MAC), i.e., C5b-9. C5b attaches to C6, followed by C6 and C7 forming complex C5b-6-7, and this complex acts as a receptor for C8 and C9. After polymerization, finally, the MAC complex is formed.
Inhibitors of the classical pathway
The complement system must be controlled to prevent unwanted host cell attacks. Inhibitors present in host tissues are present in the soluble or membrane-bound form. Decay acceleration activity (DAA) and factor I cofactor activity (CA) are the two main regulation mechanisms that accelerate the rate of dissociation of C3 convertase and factor I-mediated cleavage of C3b-C4b incapable of reforming the C3 convertase, respectively.
Some of the complement inhibitors include:
1. Soluble regulators:
- C1-Inhibitor (C1-INH): C1 is inhibited by plasma serine proteinase belonging to the serpin superfamily, also known as C1 esterase inhibitor.
- Factor I: It cleaves C3b and C4b, thus blocking the formation of C3 and C5 convertase.
- Others: C4bp, carboxypeptidase N, Factor H
2. Membrane-bound proteins:
- CD35 (Complement receptor 1 or CR1): In humans, it is present on antigen-presenting cells, erythrocytes, etc. It accelerates the decay of the C3/C5 convertase. It also acts as a co-factor for factor I in the degradation of C3b and C4b.
- CD46 (Membrane co-factor protein or MCP): It acts as a co-factor for factor I in the degradation of C3b and C4b.
- CD55 (Decay accelerating factor or DAF): It inhibits and accelerates the decay of C3 convertase.
- CD59 (protectin): It inhibits the pathway at the terminal step by inhibiting the insertion of the C9 to C5b-8 complex hence preventing MAC assembly.
- Others: CrrY
Significance/Applications of classical pathway of the complement system
- This pathway plays a key role in the opsonization and removal of nuclear debris. Bacteria and viruses are easily phagocytosed in the presence of C3b as C3b receptors are numerous on the surface of phagocytes.
- The small fragments C4a,3a, and C5a are important mediators of inflammation, while C2a is the precursor of vasoactive kinin.
- Membrane attack complex forms a pore or transmembrane channel, allowing free exchange of ions between cell and the external surrounding. The rapid influx of ions into the cell increases osmotic pressure inside the cell, which eventually bursts.
- Autoantibodies can also trigger this pathway leading to diseases like hemolytic anemia, myasthenia gravis, and bullous pemphigoid.
References
- Complement | NEJM
- Owen JA et al (2013). Kuby Immunology. 7th edition. W.H. Freeman Company. New York
- The complement system and innate immunity – Immunobiology – NCBI Bookshelf (nih.gov)
- The complement system: history, pathways, cascade and inhibitors (nih.gov)

I want introduction and definition of pathways
The notes I got from this website makes any hard topic too easy to understand and easily helps to scoe high