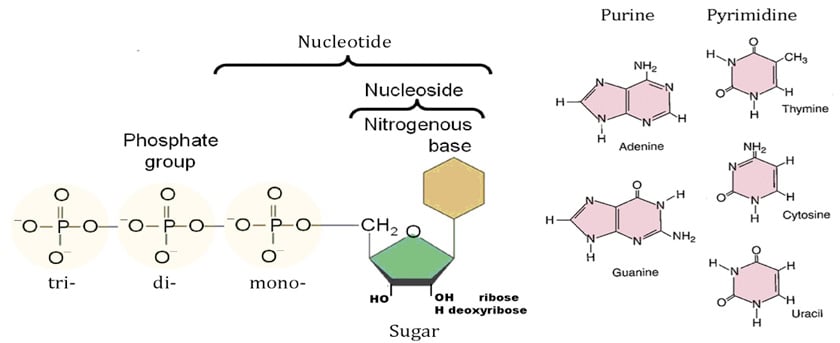
- Nucleotide is any member of the class of organic compounds in which the molecular structure comprises a nitrogen-containing unit (base) linked to a sugar and a phosphate group.
- They are monomeric units of nucleic acids and also serve as sources of chemical energy (ATP, GTP), participate in cellular signalling (cAMP, cGMP) and function as important cofactors of enzymatic reactions (coA, FAD, FMN, NAD+).
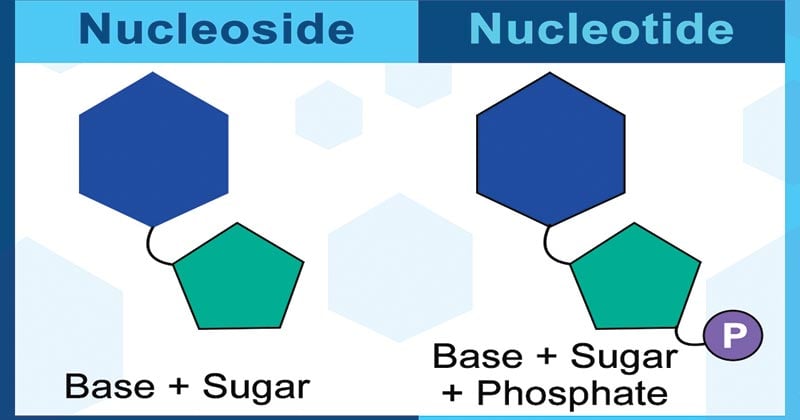
- The molecule without the phosphate group of nucleotides is called as nucleoside.
- Nucleosides are glycosylamines consisting simply of a nitrogenous base and a five-carbon sugar (either ribose or deoxyribose).
Interesting Science Videos
Structure of Nucleotides
A single nucleotide is made up of three components: a nitrogen-containing base, a five-carbon sugar (pentose), and at least one phosphate group With all three joined, a nucleotide is also termed a “nucleoside phosphate”.
Individual phosphate molecules repetitively connect the sugar-ring molecules in two adjacent nucleotide monomers, thereby connecting the nucleotide monomers of a nucleic acid end-to-end into a long chain.
Unlike in nucleic acid nucleotides, singular cyclic nucleotides are formed when the phosphate group is bound twice to the same sugar molecule, i.e., at the corners of the sugar hydroxyl groups
Nitrogenous bases
- The nitrogenous base is either a purine or a pyrimidine.
- There are five major bases found in cells. The derivatives of purine are called adenine and guanine, and the derivatives of pyrimidine are called thymine, cytosine and uracil.
- Purines include adenine and guanine and have two rings.
- Adenine has an ammonia group on its rings, whereas guanine has a ketone group.
- Pyrimidines include cytosine, thiamine, and uracil and have one ring.
- Thymine (found in DNA) and uracil (found in RNA) are similar in that they both have ketone groups, but thymine has an extra methyl group on its ring.
- Bonds between guanine and cytosine (three hydrogen bonds) are stronger than bonds between adenine and thymine (two hydrogen bonds).
Pentose Sugar
- The five-carbon sugar is either a ribose (in RNA) or a deoxyribose (in DNA) molecule.
- In nucleotides, both types of pentose sugars are in their beta-furanose (closed five-membered ring) form.
Structure of Nucleosides
- While a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups, a nucleoside has only a nitrogenous base and a five-carbon sugar.
- In a nucleoside, the base is bound to either ribose or deoxyribose via a beta-glycosidic linkage at 1’ position.
- Examples of nucleosides include cytidine, uridine, adenosine, guanosine, thymidine and inosine.
Properties of Nucleotides
Properties of purine bases
- Sparingly soluble in water
- Absorb light in UV region at 260 nm. (detection & quantitation of nucleotides)
- Capable of forming hydrogen bond
- Aromatic base atoms numbered 1 to 9
- Purine ring is formed by fusion of pyrimidine ring with imidazole ring.
- Numbering is anticlockwise.
Adenine : Chemically it is 6-aminopurine
Guanine : Chemically it is 2-amino,6-oxy purine
Can be present as lactam & lactim form
Properties of pyrimidine bases
- Soluble at body pH
- Also absorb UV light at 260 nm
- Capable of forming hydrogen bond
- Aromatic base atoms are numbered 1 to 6 for pyrimidine.
- Atoms or group attached to base atoms have same number as the ring atom to which they are bonded.
Cytosine: Chemically is 2-oxy ,4-amino pyrimidine
Exist both lactam or lactim form
Thymine: Chemically is 2,4 dioxy ,5-methyl pyrimidine
Occurs only in DNA
Uracil: Chemically is 2,4 dioxy pyrimidine
Found only in RNA
Properties of Pentose Sugars
- A pentose is a monosaccharide with five carbon atoms.
- Ribose is the most common pentose with one oxygen atom attached to each carbon atom.
- Deoxyribose sugar is derived from the sugar ribose by loss of an oxygen atom.
- The aldehyde functional group in the carbohydrates react with neighbouring hydroxyl functional groups to form intramolecular hemiacetals.
- The resulting ring structure is related to furan, and is termed a furanose.
- The ring spontaneously opens and closes, allowing rotation to occur about the bond between the carbonyl group and the neighboring carbon atom yielding two distinct configurations (α and β). This process is termed mutarotation.
Classification of Nucleotides
On the basis of the type of sugar present, nucleotides may be:
- Ribonucleotides if the sugar is ribose.
- Deoxyribonucleotides if the sugar is deoxyribose.
Classification of Nucleosides
On the basis of type of nitrogenous bases present, nucleoside derivatives may be also grouped as following:
- Adenosine nucleotides: ATP, ADP, AMP, Cyclic AMP
- Guanosine nucleotides: GTP, GDP, GMP, Cyclic GMP
- Cytidine nucleotides: CTP, CDP, CMP and certain deoxy CDP derivatives of glucose, choline and ethanolamine
- Uridine nucleotides: UDP
- Miscellaneous : PAPS (active sulphate), SAM (active methionine), certain coenzymes like NAD+, FAD, FMN, Cobamide coenzyme, CoA
Functions of Nucleotides
- The nucleotides are of great importance to living organisms, as they are the building blocks of nucleic acids, the substances that control all hereditary characteristics.
- Polynucleotides consist of nucleosides joined by 3′,5′-phosphodiester bridges. The genetic message resides in the sequence of bases along the polynucleotide chain.
- Nucleotides have a variety of roles in cellular metabolism. They are the energy currency in metabolic transactions.
- They act as essential chemical links in the response of cells to hormones and other extracellular stimuli.
- They are the structural components of an array of enzyme cofactors and metabolic intermediates.
- The structure of every protein, and ultimately of every biomolecule and cellular component, is a product of information programmed into the nucleotide sequence of a cell’s nucleic acids.
- Serving as energy stores for future use in phosphate transfer reactions. These reactions are predominantly carried out by ATP.
- Forming a portion of several important coenzymes such as NAD+, NADP+, FAD and coenzyme A.
- Serving as mediators of numerous important cellular processes such as second messengers in signal transduction events. The predominant second messenger is cyclic-AMP (cAMP), a cyclic derivative of AMP formed from ATP.
- Serving as neurotransmitters and as signal receptor ligands. Adenosine can function as an inhibitory neurotransmitter, while ATP also affects synaptic neurotransmission throughout the central and peripheral nervous systems. ADP is an important activator of platelet functions resulting in control of blood coagulation.
- Controlling numerous enzymatic reactions through allosteric effects on enzyme activity.
- Serving as activated intermediates in numerous biosynthetic reactions. These activated intermediates include S-adenosylmethionine (S-AdoMet or SAM) involved in methyl transfer reactions as well as the many sugar coupled nucleotides involved in glycogen and glycoprotein synthesis.
References
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- https://themedicalbiochemistrypage.org/nucleic-acids.php
- https://www.slideshare.net/prachandrajb/nucleotide-chemistry
- https://en.wikipedia.org/wiki/Nucleotide

Great site for specific items