Interesting Science Videos
What is Klebsiella pneumoniae?
Klebsiella pneumoniae is a Gram-negative, facultatively anaerobic, rod-shaped, non-motile, lactose-fermenting, encapsulated Gammaproteobacteria in the family Enterobacteriaceae of the phylum Pseudomonadota. It is a pathogenic species of the genus Klebsiella. It is also called “Friedlander’s Bacillus” as it was first described by German Microbiologist and Pathologist Carl Friedlander in 1882.
Klebsiella is a genus of Gram-negative, oxidase-negative, rod-shaped encapsulated members of the Enterobacterales. Currently, there are seven well-defined species of the genus Klebsiella, some of which are further classified into subspecies. All of them are ubiquitous and mostly found in the intestine of animals as commensals and found in decaying vegetation and organic wastes, sewages, contaminated soil and water bodies, etc. However, four species are frequently reported as pathogenic to humans. K. pneumoniae, K. oxytoca, K. aerogenes, and K. rhinoscleromatis.
Among these K. pneumoniae is the most important human pathogen. Although it is classified as an opportunistic pathogen, it is the most frequently reported pathogen of this genus, especially infecting hospital-admitted patients and the morbidity and mortality are high. It is responsible mainly for hospital-acquired respiratory tract infections (HA-RTIs). Community-acquired RTIs by K. pneumoniae are also common, but the prevalence is lower than nosocomial RTIs. The mortality may be up to 50% in infection by antibiotic-resistant strains and in immune-compromised patients, and the rate can be even around 100% in case of alcoholism and bacteremia.
Moreover, the rapid development of antibiotic resistance against available options is very high in K. pneumoniae. Several strains are now resistant to the antibiotic of the last resort, carbapenems. Additionally, its capacity to form a capsule and a biofilm allows it to survive and colonize in a dry habitat, like medical devices, inanimate surfaces, etc., and hence infect immune-compromised and hospitalized patients. And this is fueling the case severity and mortality rate associated with K. pneumoniae infections.
Besides its negative role in human health, K. pneumoniae is important for crop production. Several studies have shown their capacity to fix nitrogen in the anaerobic condition in the soil.
Morphology of Klebsiella pneumoniae
- Gram-negative Rod-shaped
- Arranged mostly singly, but are also seen in pairs, short chains, or rarely in the cluster
- 1.0 – 2.0 microns by 0.5 – 0.8 microns
- Non-motile
- Highly capsulated (few nonvirulent strains are non-capsulated)
- Non-sporing
- Non-flagellated
Biochemical Characteristics of Klebsiella pneumoniae
- Anaerobic and Facultative anaerobe
- Lactose fermentative
- Oxidase negative
- Catalase positive
- IMViC test = -ve, -ve, +ve, +ve (- – + +)
- Urease positive
- Nitrate positive
- TSI test = Alkaline / Alkaline (Red/Red), gas production, no H2S
Cultural Characteristics of Klebsiella pneumoniae
- Anaerobic or facultative incubation with a temperature range from 15-40°C; the optimum temperature of 35 (±2)°C
- The optimum pH requirement is 7.0 but can survive in a slightly alkaline pH range but can’t grow at acidic pH
- Non-fastidious in nutrient requirement
- Nutrient Agar and MacConkey Agar are commonly used in laboratories
Nutrient Agar = small 2 – 3 mm, dome-shaped, circular, Smooth (in fresh culture), Mucoid, greyish white, Opaque – Translucent colonies
MacConkey Agar = small 2 – 3 mm, convex, lactose fermentative, circular, Smooth, Mucoid, Opaque, Red or Pink colonies
Blood Agar = small 2 – 3 mm, non-hemolytic, greyish white, Smooth, Mucoid, Translucent – Opaque colonies
Eosin Methylene Blue Agar = small 2 – 3 mm, convex, smooth, mucoid, Pink or purplish, Opaque – Translucent colonies
Virulence Factors of Klebsiella pneumoniae
K. pneumoniae contains a wide range of cell-associated as well as extracellular virulence factors contributing to its pathogenicity. The most relevant virulence factors of K. pneumoniae with their proposed roles are summarized in the following table, viz.:
| Virulence Factors of Klebsiella pneumoniae | Roles |
|---|---|
| Capsule It is the outer polysaccharide layer covering the bacterial cell outside of the bacterial cell wall. | – Helps in evading phagocytosis and protecting the bacterial cells – Facilitates biofilm formation and attachment – Protects the invading bacteria from the lethal actions of the host’s complement system – Confer resistance against several antibodies and antimicrobial peptides – Provide resistance against antibiotics – Rarely suppress and attack immune cells – Acts as a physical barrier against physical stresses and chemicals |
| Fimbriae They are small projections that help in bacterial adhesion and colonization. Two types of fimbriae ‘Type 1’ and ‘Type 3’are important virulence factors. | – Type 1 fimbriae are responsible for the formation of bacterial communities and cell adhesion – Type 3 fimbriae are responsible for the formation of biofilm and help in bacterial attachment on the host cell and medical devices. |
| Porins They are outer membrane protein channels that regulate bacterial permeability. OmpK 35, OmpK 36, and OmpK 37 are the most important porins produced by K. pneumoniae. | – OmpK 36 is found to disturb the activation of the complement system allowing bacteria to escape the host’s immune response. – Facilitates biofilm formation and attachment – Provide resistance against antibiotics |
| Lipopolysaccharides (LPS) | LPS are responsible for: – Adherence – Resistance against phagocytes – Activation of the complement factors and protect the bacteria against the host’s complement killing – Conferring serum resistance to the bacteria – Helps in colonization and dissemination inside the host’s internal organs – Helps in the formation of biofilm – Confer antibiotic resistance |
| Outer Membrane Proteins (OMPs) They are outer membrane protein vesicles secreted by the outer membrane of the bacterium for transportation. | OMPs are responsible for: – Preventing activation of epithelial cells in the host’s respiratory tract and protecting from the inflammatory responses – Protects against neutrophils – Acquisition of antibiotic resistance |
| Siderophore It is the system present in the bacterial cell to meet up their iron requirement. | – Promotes bacterial iron acquisition and survival inside the host’s body |
| Type 6 Secretory System | – Helps in injecting effector proteins inside the host’s cells to destroy the host cells and limit their actions against the invading bacterial cells |
Clinical Manifestation of Klebsiella pneumoniae
K. pneumoniae is a normal flora of the intestinal tract, mouth, and rarely skin. It is responsible for both hospital-acquired and community-acquired infections; however, the prevalence of hospital-acquired infections is quite higher. Besides pneumoniae, K. pneumoniae is reported as a common pathogen infecting the Urinary tract, meninges, liver, wounds, and blood. It is a prominent pathogen accounting for over 10% of HAIs.
Biofilm formation and antibiotic resistance against most of the available antibiotics have increased the morbidity and mortality of K. pneumoniae infections.
K. pneumoniae is frequently associated with the following clinical syndromes:
- Respiratory Tract Infections (Pneumonia)
K. pneumoniae is the most common bacterial pathogen causing pneumonia after Streptococci. It is held responsible for about 12% of hospital-acquired pneumonia. It is responsible for Ventilator-associated pneumonia (VAP), and pneumonia in patients in intensive care units (ICUs). Besides, it is also responsible for a large portion of community-acquired pneumonia, particularly in alcoholics. Pneumonia by K. pneumoniae leads to necrosis of lung tissue and produces the characteristic mass of sputum with mucous, cell debris, and blood called the “currant jelly”.
- Urinary Tract Infections
UTI is another common disease caused by K. pneumoniae, especially in hospitalized patients with an indwelling urinary catheter. Catheterized patients and kidney disease patients are at high risk of UTI by K. pneumoniae. Community-acquired UTIs are also commonly reported. K. pneumonia-associated UTIs cover about 6 – 17% of all UTI cases.
- Soft Tissue and Skin Infection
K. pneumoniae is found to infect surgical wounds and injured skin. It results in cellulitis, necrotizing fasciitis, and myositis. They are also reported to cause wounds in the lining of the esophagus, stomach, and intestines.
- Blood Stream Infection (BIs) and Septicemia
K. pneumoniae is responsible for 4 – 15% of all septicemia cases and about 3 – 20% of neonatal septicemia. Studies have shown that BIs by Klebsiella pneumoniae are highly due to transmission of the pathogen from the lung to the bloodstream, so secondary bacteremia and septicemia are more common than primary ones. The mortality rate may rise above 70% in infection by resistant strains and in patients with other underlying medical conditions.
- Central Nervous System Infections (Meningitis)
Klebsiella pneumoniae-associated meningitis is often seen in hospitalized patients with underlying medical conditions like diabetes and liver cirrhosis.
- Pyogenic Liver Abscess
It is more prevalent in people with diabetes, alcohol addiction, and people with prolonged antibiotic therapy. Pus is formed inside the liver along with swelling and inflammation in the surrounding. If left untreated, it can even cause liver damage.
- Endophthalmitis
Patients with K. pneumoniae BIs sometime suffer from Endophthalmitis which can even lead to permanent blindness.
What are Carbapenems?
- Broad-spectrum β-Lactam antibiotics are used against serious infections, usually against those caused by multidrug-resistant bacterial pathogens.
- Can resist the effects of β-Lactamase enzymes
- Susceptible to ‘carbapenemases’
- Beta-lactam antibiotics have sulfur at the C-1 position and a double bond in between the C-2 and C-3 of the Beta-lactam ring with the side chains arranged in the trans position.
- Mode of action: Binding to the PBPs (penicillin-binding proteins), hence inhibiting bacterial cell wall formation.
- Carbapenems currently in use are “IMIPENEM”, “MEROPENEM”, “ERTAPENEM”, and “DORIPENEM”.
- Called “the antibiotics of last resort”
What are Carbapenem-Resistant Organisms (CROs)?
- Bacteria that have developed one or more mechanisms to resist themselves the action of carbapenems are carbapenems-resistant organisms.
- They are labeled as organisms of “urgent threat requiring urgent countermeasures” by the US Center for Disease Control and Prevention (CDC), and “priority one pathogen” by the World Health Organization (WHO).
Some most serious carbapenem-resistant bacteria are:
- Carbapenem-resistant Enterobacteriaceae (CRE) [Carbapenem-resistant Klebsiella pneumoniae is the most important among them]
- Carbapenem resistant Acinetobacter baumannii (CRAB)
- Carbapenem resistant Pseudomonas aeruginosa (CRPA)
What is Carbapenem-resistant Klebsiella pneumoniae (CRKP)?
Carbapenem-Resistant Klebsiella pneumoniae (CRKP) are the Klebsiella pneumoniae bacteria that have developed several mechanisms to protect themselves from the inhibitory or bactericidal action of carbapenems.
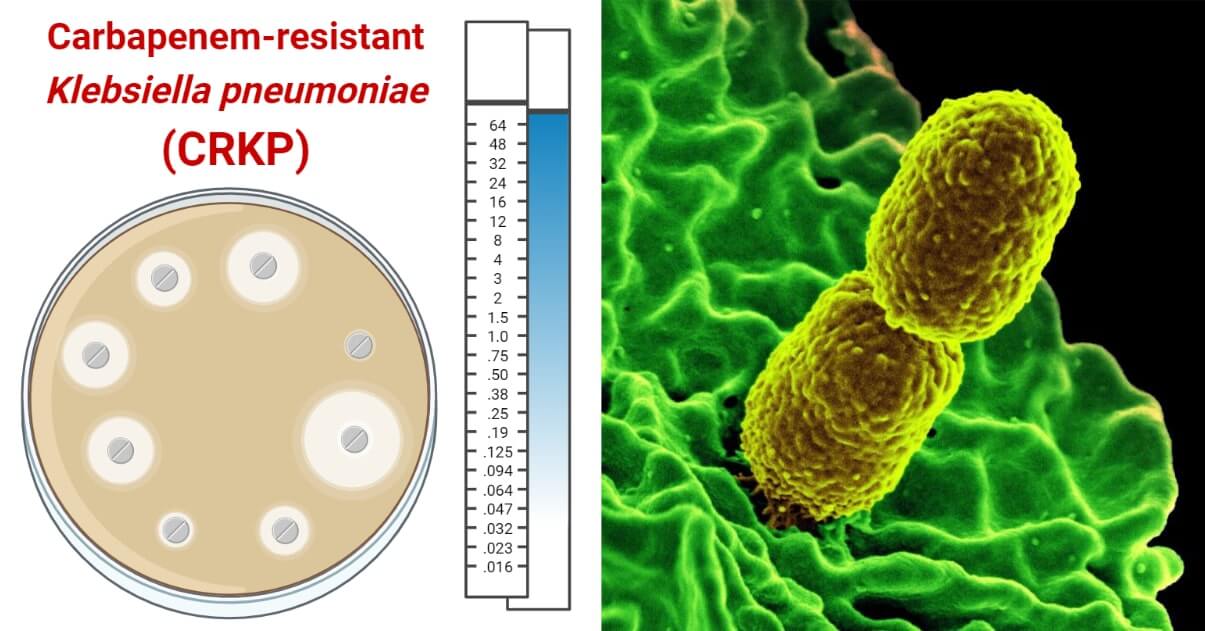
- CRKP is the most concerned member of the CRE group. CRE is labeled as “an urgent threat” by the US CDC in 2019 and “a priority 1 – critical pathogen” by the WHO since 2017 and CRKP is the most important pathogen in the group with a major threat in public threat. It is also a member of the ESKAPE group including MDR pathogenic bacteria associated with most of the HAIs.
- Infections by CRKP have higher case severity and mortality than those caused by sensitive strains of K. pneumoniae. The mortality rate may be from 23% to 75%.
Risk Factors for Carbapenem-resistant Klebsiella pneumoniae Infections
- Hospitalized patients and patients with the weakened immune system
- Catheterized patients with indwelling urinary catheters
- Patients with intubation and ventilation
- Patients with underlying medical conditions like diabetes mellitus, chronic kidney diseases, urinary obstruction, heart disorder, patients using steroids and anti-pseudomonal penicillins, and patients under prolonged antimicrobial therapy
- Transplant recipient
- Patients with recent surgery and open wound patients
- Chronic Obstructive Pulmonary Disease (COPD) patients
- Chronically and acutely ill patients
Mechanisms of Resistance Against Carbapenems by CRKP
K. pneumoniae has developed several mechanisms of resistance against carbapenems. Some of the well-studied resistant mechanisms are:
a. Enzymatic Modification of Antibiotics
CRKP is able to produce various carbapenemase enzymes that can hydrolyze the β- lactam ring of carbapenems, and hence provide them resistance against the deleterious actions of carbapenems.
Carbapenemases found to be produced by K. pneumoniae are listed below.
- The “K. pneumoniae carbapenemase (KPC) β- lactamases” are exclusively reported in CRKP. KPC β– Lactamases production is the most important strategy developed by CRKP to escape from the actions of carbapenems. Since its first report of K. pneumoniae in the late 1990s from North Carolina USA, it is now reported from all over the world. KPC-2 and KPC-3 type β- lactamases are most frequently reported in K. pneumoniae.
- The “Metallo –β – Lactamases (MBLs)” are another important carbapenemases produced by many of the clinical isolates of K. pneumoniae. New Delhi Metallo – β– Lactamase – 1 (NDM – 1) , Imipenem-resistant Pseudomonas (IMP) Metallo-β-lactamase group and Verona integron-related Metallo-β-lactamase (VIM-1 and VIM-2) groups are the most frequently reported MBLs types in CRKP.
- The class – D β- lactamases like “Oxacillinase (OXA β- lactamases)”, mainly OXA – 48 type and its derivatives like OXA – 181, OXA – 204, and OXA – 232 are also produced by K. pneumoniae.
b. Modification of Porins
Modification in the outer membrane porin OmpK35, OmpK36, and OmpK37 is reported in CRKP. OmpK35 is highly downregulated and they are very few to completely absent in CRKP strains.
c. Over Expression of Efflux Pumps
Over-expression of the efflux pumps is another mechanism developed by K. pneumoniae to expel carbapenems from their cytoplasm and confer resistance against them. RND type efflux pump family is the most important efflux pump that is responsible for resistance against carbapenems. Among the RND types, AcrAB and OqxAB are the most potent efflux pumps.
d. Capsule
The acidic polysaccharide capsule of K. pneumoniae also aids a little in conferring resistance against carbapenems by acting as a physical barrier and diluting the antibiotics.
e. Biofilm Formation
Biofilm formation is another general mechanism that confers resistance against diverse antimicrobials including carbapenems. It acts as a physical barrier and prevents carbapenems from entering the cell.
Epidemiology of Carbapenem-resistant Klebsiella pneumoniae
Since its first report in Northern USA in the early 21st century, CRKP is now a global burden. Within the last decade, carbapenem-resistant strains of K. pneumoniae have spread worldwide and are common pathogens causing HAIs. USA, Latin America, South American countries, European regions, Southeast Asian countries, China, Mediterranean countries, and even African countries have an endemic prevalence of CRKP. Their prevalence in hospital settings is between 20 and 40% in endemic regions.
Identification of Carbapenem-resistant Klebsiella pneumoniae
The phenotypic identification method is commonly used in diagnostic and research laboratories. For more precise identification (study mechanisms and genetics) and research studies, molecular methods are used.
A biochemical testing algorithm is first used in the laboratory to identify the isolate as K. pneumoniae and then the identified are tested for carbapenem resistance.
Biochemical Algorithm For Identification of Klebsiella pneumoniae
- Gram-negative Rod
- Non-motile
- Capsulated
- Oxidase – ve
- IMViC = – – + +
- Nitrate +ve
- Urease reducing (+ ve)
- TSI = Y/Y (Acidic / Acidic), Gas + ve and H2S –ve
- Growth on KCN +ve
Confirmation of Carbapenem Resistance in Klebsiella pneumoniae (CRKP)
Phenotypic Confirmation of CRKP by Antimicrobial Sensitivity Testing and Calculation of Zone of Inhibition
Phenotypically, CRKP can be confirmed by performing Antimicrobial Sensitivity Test using carbapenems. If the size of the zone of inhibition is less than recommended or in the level of resistance, then we can confirm the isolate to be CRKP.The zone size interpretative chart for carbapenems against K. pneumoniae according to the CLSI standard is presented below.
| Zone Size (in mm) For: | Zone Size (in mm) For: | Zone Size (in mm) For: | |
| Carbapenem Antibiotics | Sensitive | Intermediate | Resistance |
| Doripenem (DOR 10 mcg) | ≥23 | 20 – 22 | ≤19 |
| Ertapenem (ETP 10 mcg) | ≥23 | 19 – 21 | ≤18 |
| Imipenem (IMP 10 mcg) | ≥23 | 20 – 22 | ≤19 |
| Meropenem (MRP 10 mcg) | ≥23 | 20 – 22 | ≤19 |
The zone size interpretative chart for carbapenems against K. pneumoniae according to the EUCAST standard is presented below.
| Zone Size (in mm) For: | Zone Size (in mm) For: | Zone Size (in mm) For: | |
| Carbapenem Antibiotics | Sensitive | Intermediate | Resistance |
| Doripenem (DOR 10 mcg) | ≥22 | 17-21 | ≤16 |
| Ertapenem (ETP 10 mcg) | ≥25 | – | ≤25 |
| Imipenem (IMP 10 mcg) | ≥22 | 18-21 | ≤17 |
| Meropenem (MRP 10 mcg) | ≥22 | 17-21 | ≤16 |
The common methods for phenotypic detection of carbapenemase production in CRKP include:
- Modified Hodge Test
- Carba NP Test
- Modified Carbapenem Inactivation Method / Carbapenem Inactivation Method
- Blue Carba Test
- EDTA Inhibition Method
- Boronic Acid Inhibition Test
- mCIM/eCIM Test
- Double Disk Synergy Test (using 10 μg 0.5 M EDTA disk and 10 μg imipenem or meropenem disk) and Combined Disk Synergy Test (using one imipenem or meropenem disk with EDTA and another without EDTA)
- Chromogenic media: currently two chromogenic agar mediums are used to separate carbapenemase producers from non-producers, they are: CHROMagar-KPC, and Brilliance CRE agar.
Molecular Confirmation of CRAB
- Polymerase Chain Reaction (PCR)
- Xpert Carba-R molecular test
- Multiplex PCR
- Duplex multiple cross displacement amplification combined with lateral flow biosensor (MCDA-LFB) method
- DNA Microarray
- Verigene Gram-negative blood culture assay
- Check KPC ESBL microarray
- BioFire Film Array Method
- Whole Genome Sequencing
- Mass Spectroscopy
Prospective Treatment Options for CRKP Infections
Carbapenems are “the antibiotic of last resort”, therefore active treatment options available for CRKP are very limited. There is no precise treatment regimen designed, but several combination therapies and high-dosage antibiotics are practiced as an appropriate and alternative therapy in clinical settings. There is no known optimal treatment option; combination therapy is the most effective option. Some commonly used therapeutics are:
- Polymyxins (Colistin and Polymyxin B)
- Tigecycline
- Fosfomycin-tigecycline
- Tigecycline-colistin-aminoglycoside
- Ceftolozane-tazobactam,
- Ceftazidime-avibactam,
- Imipenem-cilastatin-relebactam,
- Cefiderocol,
- Meropenem – Vaborbactam
- A single dose of an aminoglycoside
- Ampicillin – Sublactam (Cefoperazone – Sulbactam)
- Carbapenem + aztreonam (in case of NDM, VIM and IMP producers)
- Colistin + aminoglycosides/carbapenems/aztreonam
- Rifampin + tigecycline/colistin
- Avibactam-ceftazidime
- Plazomicin
Future/Alternative Treatment Options Under Study
- Cefederocol
- Delafloxacin (Baxdale)
- Nanoparticle therapy
- Phage therapy
- Nucleic acid-based antibiotics
- Engineered endolysins
- Vaccines
Prevention and Control Measures from CRKP Infections
- Infection prevention and control strategies; such as: Hygiene maintenance, sanitation, contact precaution, patient isolation,
- Tracing, isolating, and treating carriers
- Strict and compulsory hand hygiene practice in hospital settings
- Frequent checking of isolates for carbapenemase production and quick transfer of the information to the concerned authority in the hospital
- Proper maintenance of sanitation in patient rooms and proper sterilization of hospital settings
- Rigorous practice of proper and adequate protective measures by hospital staff
- Sterilization of medical devices before administration
- Effective surveillance system
- Maintaining a sterile environment in pre- and post-operation wards, operation theater, ICUs, and ventilators
- Rational use and prescription of antibiotics
- No antimicrobials without prescription
- Antimicrobial therapy stewardship
References
- Klebsiella species – GlobalRPH
- Klebsiella species: guidance, data and analysis – GOV.UK (www.gov.uk)
- Klebsiella | Description, Species, & Infection | Britannica
- Morphology & Culture Characteristics of Klebsiella pneumoniae (paramedicsworld.com)
- Ashurst JV, Dawson A. Klebsiella Pneumonia. [Updated 2022 Feb 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519004/
- Albertí, S., Marqués, G., Hernández-Allés, S., Rubires, X., Tomás, J. M., Vivanco, F., & Benedí, V. J. (1996). Interaction between complement subcomponent C1q and the Klebsiella pneumoniae porin OmpK36. Infection and Immunity, 64(11), 4719-4725. https://doi.org/10.1128/iai.64.11.4719-4725.1996
- García-Sureda, L., Juan, C., Doménech-Sánchez, A., & Albertí, S. (2011). Role of Klebsiella pneumoniae LamB Porin in Antimicrobial Resistance. Antimicrobial Agents and Chemotherapy, 55(4), 1803-1805. https://doi.org/10.1128/AAC.01441-10
- Jasim, Saade & Ayad, Sumaya & Hashoosh, Sarah & Saleh, Raed. (2020). Virulence Factors of Klebsiella pneumoniae Isolates from Iraqi Patients. Systematic Reviews in Pharmacy. 11. 2020. 10.31838/srp.2020.6.129.
- Klebsiella Pneumoniae Infection: Symptoms, Causes, Treatment (healthline.com)
- Klebsiella pneumoniae in Healthcare Settings | HAI | CDC
- Zhu, J., Wang, T., Chen, L., & Du, H. (2021). Virulence Factors in Hypervirulent Klebsiella pneumoniae. Frontiers in Microbiology, 12. https://doi.org/10.3389/fmicb.2021.642484
- (2020). Virulence evolution, molecular mechanisms of resistance and prevalence of ST11 carbapenem-resistant Klebsiella pneumoniae in China: A review over the last 10 years. Journal of Global Antimicrobial Resistance, 23, 174-180. https://doi.org/10.1016/j.jgar.2020.09.004
- Podschun, R., & Ullmann, U. (1998). Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clinical Microbiology Reviews, 11(4), 589-603. https://doi.org/10.1128/cmr.11.4.589
- Liu, P., Li, X., Luo, M., Xu, X., Su, K., Chen, S., Qing, Y., Li, Y., & Qiu, J. (2018). Risk Factors for Carbapenem-Resistant Klebsiella pneumoniae Infection: A Meta-Analysis. Microbial Drug Resistance, 24(2), 190-198. https://doi.org/10.1089/mdr.2017.0061
- Pitout, D. D., Nordmann, P., & Poirel, L. (2015). Carbapenemase-Producing Klebsiella pneumoniae, a Key Pathogen Set for Global Nosocomial Dominance. Antimicrobial Agents and Chemotherapy, 59(10), 5873-5884. https://doi.org/10.1128/AAC.01019-15
- Tzouvelekis, L. S., Markogiannakis, A., Psichogiou, M., Tassios, P. T., & Daikos, G. L. (2012). Carbapenemases in Klebsiella pneumoniae and Other Enterobacteriaceae: an Evolving Crisis of Global Dimensions. Clinical Microbiology Reviews, 25(4), 682-707. https://doi.org/10.1128/CMR.05035-11
- Reyes, J., Aguilar, A. C., & Caicedo, A. (2019). Carbapenem-Resistant Klebsiella pneumoniae: Microbiology Key Points for Clinical Practice. International Journal of General Medicine, 12, 437-446. https://doi.org/10.2147/IJGM.S214305
- Yigit, H., Queenan, A. M., Anderson, G. J., Domenech-Sanchez, A., Biddle, J. W., Steward, C. D., Alberti, S., Bush, K., & Tenover, F. C. (2001). Novel Carbapenem-Hydrolyzing β-Lactamase, KPC-1, from a Carbapenem-Resistant Strain of Klebsiella pneumoniae. Antimicrobial Agents and Chemotherapy, 45(4), 1151-1161. https://doi.org/10.1128/AAC.45.4.1151-1161.2001
- Ni, R. T., Onishi, M., Mizusawa, M., Kitagawa, R., Kishino, T., Matsubara, F., Tsuchiya, T., Kuroda, T., & Ogawa, W. (2020). The role of RND-type efflux pumps in multidrug-resistant mutants of Klebsiella pneumoniae. Scientific Reports, 10. https://doi.org/10.1038/s41598-020-67820-x
- Weng, X., Shi, Q., Wang, S., Shi, Y., Sun, D., & Yu, Y. (2020). The Characterization of OXA-232 Carbapenemase-Producing ST437 Klebsiella pneumoniae in China. The Canadian Journal of Infectious Diseases & Medical Microbiology = Journal Canadien des Maladies Infectieuses et de la Microbiologie Médicale, 2020. https://doi.org/10.1155/2020/5626503
- Yu, X., Zhang, W., Zhao, Z., Ye, C., Zhou, S., Wu, S., Han, L., Han, Z., & Ye, H. (2019). Molecular characterization of carbapenem-resistant Klebsiella pneumoniae isolates with focus on antimicrobial resistance. BMC Genomics, 20. https://doi.org/10.1186/s12864-019-6225-9
- Guidelines11FinalPrint (who.int)
