Spread Plate Method is one of the widely used culture techniques in microbiology laboratories due to its ease and simplicity. This method is suitable for aerobic and facultative aerobic microorganisms. It is an easy, simple, and economical method; however, it requires the sample to be in liquid or suspension.
Interesting Science Videos
What is Spread Plate Method?
The spread plate method is a microbiological laboratory technique for isolating and counting the viable microorganisms present in a liquid sample by spreading a certain volume of the sample over an appropriate solidified culture media.
Following the incubation, in a successful spread plate, there will be the formation of evenly distributed discrete colonies all over the surface of the culture media.
This technique is used for isolating and counting the total number of viable microorganisms (i.e. calculating the colony-forming units per mL (CFU/mL) in the given sample. It is also for propagating the old culture and mass producing them. It can be used for all of the culturable bacteria and fungi.
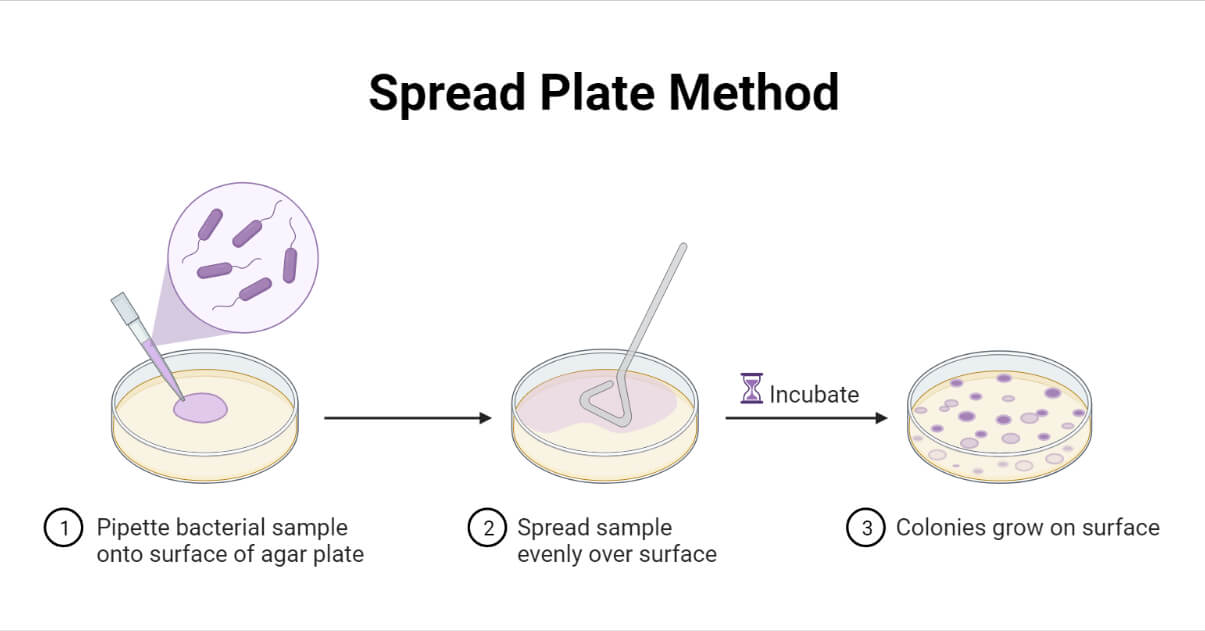
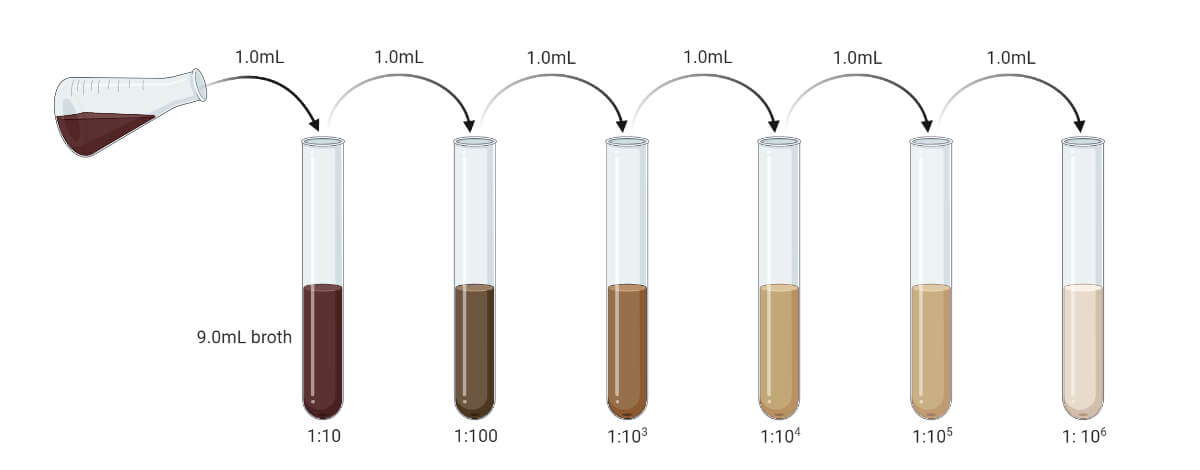
The sample in the spread plate method must be liquid or in suspension. Before plating, the samples are serially diluted. If the objective is to count the CFU/mL then the sample must be diluted to make the microbial load in the sample between 20 – 300 CFU/mL (suitable colony counting range is 20 – 200, some consider it to be 30 – 300, and in average it is taken as 25 – 250). This can be obtained by pilot test or by using a different range of diluted samples. If the sample is solid or semisolid, it must be first emulsified and then serially diluted to reduce microbial load up to the permitted range.
0.1 mL of the sample (0.1 to 0.2 mL) is pipetted over the center of the solidified agar medium and evenly spread over the surface of the medium. The plates are incubated under the optimum condition following which the numbers of colonies are counted. If the colonies are uncountable or fused or more than 300 CFU/mL or less than 20 CFU/mL, it is recommended to repeat the process for getting the optimum count.
Objectives of Spread Plate Technique
- To isolate the microorganisms from the liquid specimen (or suspension)
- To calculate viable microbial load by counting colony formation unit (CFU) per mL
- To isolate the pure culture of microorganisms from a mixed population
- To isolate microorganisms in discrete colonies in order to study their colony characters
- To obtain sufficient growth for conducting antimicrobial sensitivity testing and biochemical studies
Principle of Spread Plate Method
When a diluted liquid specimen containing one or more microorganisms, same or different species, is spread over a suitable solid agar media, each of the viable microorganisms will multiply forming a separate colony. These colonies can be counted and expressed in terms of the CFU/mL which can be used to calculate the microbial load in the sample.
A certain volume of the diluted sample, mostly 0.1 mL (0 to 1 mL can be used), is dispensed over the surface of a pre-sterilized solid medium. Usually, a bent glass rod or swab or glass beads are used to spread the sample. Following the incubation for usually 24 – 48 hours at 37°C, the viable microorganisms in the sample will grow into discrete visible colonies on the surface of the medium. The visible colonies can be counted and CFU/mL can be calculated using the following formula;
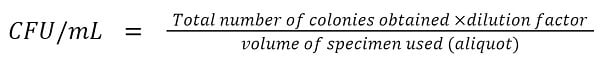
Requirements for Spread Plate Method
- Liquid Specimen (or suspension of the solid sample)
The sample must be either in liquid form or in suspension form. The solid sample must be dissolved in a suitable solvent. The solvent must not show any inhibitory or growth-promoting activity to any of the microorganisms. Also, it must not react to any component of the culture media.
The sample must be diluted to an appropriate concentration so that we can get a well-isolated 20 – 300 CFU/mL per plate after the incubation.
- Pre-solidified Suitable Solid Culture Media Plates
Appropriate culture media which supports the growth of all desired or probable microorganisms in the specimen must be used. The media must be solidified properly before spreading the diluted sample. The media can be prepared and stored at 4°C for future use. If freshly prepared, make sure the media is completely solidified.
- Test Tubes
Sterile test tubes are used to serially dilute the sample.
- Sterile Distilled Media (or Sterile Broth)
The sample must be serially diluted using sterile distilled water or sterile broth. They are also used for dissolving the solid or semi-solid sample.
- Micropipette
A micropipette of 0.1 mL or 1 mL capacity is required for measuring the sample during serial dilution and sample inoculation. A sterile graduated pipette can be used instead of the micropipette.
- Spreaders
Spreaders are the tools that are used to spread the inoculum over the surface of the agar medium. Bent glass rods, glass beads, and cotton swabs are used as a spreader.
- Bent glass rod: it is a glass rod that is bent at one end forming either “L” or “J” shaped or forming a closed triangle-like shape at one end. It is often called a “dolly rod”. It is the most commonly used spreader. Metallic rod is not preferred because their surface can be delaminated and they take a longer period to cool down. Glass rods are with smooth surface and can be easily made in a lab and also they cool down quickly.
- Glass beads: smooth tiny glass beads, usually of 4 mm diameter, are used to spread the inoculum in the “Copacabana method”. The sample is dispensed on the surface of the agar medium. Sterile glass beads are placed over the medium and the whole plate is shaken so that the beads distribute the sample evenly all over the agar surface.
- Sterile cotton swab: a sterile swab may be used, but it is not recommended when we need to count CFU/mL because some microorganisms may be trapped on the sample that will be absorbed in the swab.
- Ethanol (70%)
70% ethanol is used as a chemical sterilizer to sterilize the spreaders (except cotton swabs).
- Bunsen Burner
It is used to flame the glass spreader in order to sterile them. Besides it can also be used to make a sterile working zone.
- Other Laboratory Facilities
Procedure of Spread Plate Method
The general procedure of the spread plate method can be summarized as:
- Arrange all the requirements, put on the PPE, sterilize the work surface, and allow all the samples and media to come to room temperature if were refrigerated.
- Sample preparation
- Liquid samples must be serially diluted to reduce the microbial load to the range of 20 – 300 CFU/mL. (If the sample is assumed to be sterile or the expected microbial load is very low, we can escape the dilution. The prior pilot test may give an exact value. You can prepare serial dilution up to 10-10 and use different dilutions.)
- If the sample is solid or semisolid, dissolve it with a suitable solvent to prepare its suspension. The suspension then should be serially diluted to reduce the microbial load at the desirable range. (Generally, 1 gm sample is mixed with 9 ml of solvent to get the concentration of 10-1 gm/mL.)
- Arrange the spreader. The glass rod must be sterilized. For this dip the rod in 70% ethanol solution and flame it over a Bunsen burner. Let the rod cool. (You can check if the rod is cold enough or not by touching a corner of solidified media. If you heard a sizzling sound or if the media melts, then cool the rod further.)
Likewise, the beads can be put in a bottle or beaker and then autoclaved to sterilize them.
- Label the plate at its bottom edge with the dilution factor, date, name, sample ID, and other required information.
- The spreading can be done by either of the following two methods, viz.:
Spreading with a bent glass or metal rod
- Open the lid of the plate and dispense 0.1 mL of the diluted sample in the center of the Petri plate using a calibrated pipette or micro-pipette.
- Using a sterile bent glass rod uniformly spread the sample all over the plate.
- If you are using a turntable, spin it slowly and then hold your spreader gently on the surface of the media touching the sample, and gradually spread the sample uniformly all over the surface of the media. Moving the rod back and forth will allow you to spread the sample.
- If you are performing it manually, hold the plate in your left hand (or in your right if you are left-handed) or you can also put it still on the bench. You must move the spreader in either a circular path or in a back and forth motion to spread the sample evenly. At last, move the spreader in a circular motion around the edge of the plate to make sure that the sample is spread even at the corner.
- Put on the lid, leave the plate in an upright position and allow the sample to be absorbed for around 5 minutes. Then incubate the plate in an inverted position.
Spreading with glass beads: “Copacabana Method”
- Open a portion of the lid of a Petri plate with your thumb and index finger and dispense about 10 -12 sterile glass beads.
- Dispense 0.1 mL of the diluted sample in the center of the Petri plate using a calibrated pipette or micro-pipette.
- Close the lid and shake the plate in a horizontal motion so that the beads spread the sample evenly across the surface of the agar medium. Repeat the shaking 6 – 7 times.
- Rotate the plate clockwise or counterclockwise by 60° and again shake the plate in a horizontal motion 6 – 7 times.
- Once again, rotate the plate in the same direction by 60° and shake the plate in a horizontal motion 6 – 7 times. (By this time, the sample will be spread uniformly, but if you feel that the sample is not distributed evenly, you can repeat the shaking process.)
- Leave the plate in an upright position and allow the sample to be absorbed for around 5 minutes.
- Discard the beads in a container with disinfectant (10% chlorine bleach, ethanol, etc.)
- Incubate the plate in an inverted position.
Result Interpretation of Spread Plate Method
- Following the incubation, the plates are looked for the development of discrete colonies. Each colony will account for one viable microbial cell or one colony-forming unit.
- If the morphology of all the isolated colonies is the same, it indicates that the specimen contained only one type of microbial genera. However, there may be different genera or species producing similar types of colonies. So, perform a further test for their identification before confirming the presence of only one type of microorganism.
- If the morphology of isolated colonies is different, then we can conclude that the sample contained more than one type of genera or species of microorganism. They can be purified by sub-culturing each colony on a separate culture media plate using the streak plate method.
- Count the colonies and calculate CFU/mL by using the formula:
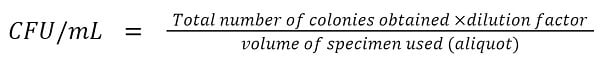
This will give the total number of viable microbial cells present in the given sample.
- For optimum count, the number of colonies must be between 20 – 300 CFU/mL. Beyond this limit, the whole procedure must be repeated. If the number of the colony is less than 20, it is suggested to use the sample of lower dilution, whereas, if the total number of colonies exceeds 300, it is suggested to use the sample of higher dilution on successive repeats.
- If the colonies are fused or the whole plate is covered with a single colony, then report as “too numerous to count” (TNTC) and repeat the process of taking the sample at a higher dilution.
Precautions during Spread Plate Technique
- Follow proper safety protocols. Treat every unknown or clinical specimen as hazardous and follow safety accordingly. Make sure that all the tools and glass wares are sterile. The water or broth used in serial dilution must be sterile.
- Make sure to sterilize the glass rods by dipping them in 70% alcohol and flaming them before and after their use to spread a specimen. Glass beads can be sterilized by autoclaving. The spreading beads or the rod must be at or below 37°C. Don’t use them immediately after flaming; let them cool.
- The solvent used to dissolve the solid sample must be sterile and must not have any growth-supportive or inhibitory effect against any microorganism.
- The sample must be diluted enough so that the viable microbial load falls between 20 – 300 CFU/mL. Above this range, it will be difficult to count the colony and the colonies may fuse together. Below this range, the result is reported as not significant. The process must be repeated under the same condition if the colony count is below 20 CFU/mL or above 300 CFU/mL.
- The sample must be 0.1 mL (0 to 1 mL is the permitted range) for spreading. If the sample is 1 mL or more, then there will be fused colonies, and also the sample may not be absorbed by the media leaving it to float on the surface.
- Media should be properly solidified before use. If it is refrigerated, allow it to come to room temperature. Appropriate media selection is necessary for proper and complete isolation.
- Check for any growth or presence of water droplets on the surface of the media before inoculation.
- Accurate measurement of water for serial dilution and accurate measurement of sample for inoculation is very important. Hence, always use a micropipette or calibrated pipette.
- Labeling each Petri plate with an accurate dilution factor is necessary in order to make an accurate calculation of the microbial load following the incubation. Inoculate each plate with the specific specimen whose concentration corresponds to the labeled dilution factor on the plate.
- Incubate the plate in an inverted position in appropriate condition. Check the plate by 24 hours of incubation, because if delayed over-growth can occur and colonies may fuse making the plate difficult to read. If no growth after 24 hours, incubate it for the next 24 – 48 hours (or more based on probable microorganism) before reporting no growth and discarding.
Applications of Spread Plate Technique
- Used to isolate bacteria and fungi from a given sample
- Used in antimicrobial sensitivity testing, and enrichment and screening experiments.
- Used to calculate the number of viable microorganisms (i.e. calculate CFU/mL) in a sample
- Used in food industries, pharmaceutical industries, soil studies, etc. in order to isolate and enumerate spoilers or contaminants to check for the quality of the products.
- Used to mass culture the stock culture or fresh specimen
- Used in clinical laboratories to inoculate the clinical specimens
- Used to study growth curves, metabolic activities, and biochemical features of microorganisms, and also the effect of environmental factors on them.
- Used in separating pure culture from a mixed culture
- Used in generating discrete and pure colonies in order to study colony characters, genetic features, and other biochemical features of the isolates.
Advantages of Spread Plate Technique
- It is a simple, easy, and quick method of culturing microorganisms.
- A very low microbial load can be detected.
- The colony morphology of a microorganism can be studied by this method. The size of colonies produced when cultured by this method will be larger than those produced by the sample species using the pour plate method.
- It is a qualitative as well as quantitative isolation method which facilitates isolation as well as an enumeration (i.e. calculation of CFU/mL) of microorganisms.
- It can be used in performing Kirby Bauer antimicrobial sensitivity testing.
- Any clinical or industrial or environmental samples can be used unless it is in a liquid state or can be dissolved to prepare a suspension.
- It is used in preparing and maintaining stock culture.
- It is the most appropriate method for culturing the aerobic microorganism.
- Even the syntrophic bacteria can be isolated because they both can be grown in a single plate nearby with distinct colonies of each type.
- There is very least chance of contamination if one used sterile glass beads as a spreader. This is because all the action will be done by closing the lid of the culture plate.
Limitations of Spread Plate Technique
- It requires extra tools like a spreader.
- It needs the sample to be in liquid or suspension form and needs to be serially diluted, hence; it is a little complex process.
- Solid or semisolid samples must be suspended prior to inoculation. It is very difficult if the sample is not easily soluble.
- It doesn’t support sufficient growth of Microaerophiles and anaerobes.
- It is unsuitable if the microbial load in the sample is too high. So, the sample must be serially diluted to reduce the microbial load at 20 – 300 CFU/mL. To get this dilution range, we may even need to do pilot testing.
- There is a chance of gouging the media during spreading, especially while using glass rod by one with not enough skill, and if the media is not properly solidified.
- Needs to sterilize the beads or glass rods after spreading each plate. If the sterilization is not enough, then there is a higher chance of cross-contamination.
- It needs specific media pre-solidified before the work. Hence, either we need prior information about probable microorganisms in the sample, or we have to have different types of media.
References
- Sanders E. R. (2012). Aseptic laboratory techniques: plating methods. Journal of visualized experiments : JoVE, (63), e3064. https://doi.org/10.3791/3064
- Textbook of Microbiology and Immunology (2012), 2nd Editions. Subah Chandra Parija. ISBN: 978-81-312-2810-4
- Practical Handbook of Microbiology, 2nd Edition. Edited by Emanuel Goldman and Lorrence H. Green. CRC Press. Taylor & Francis Group. 6000 Broken Sound Parkway NW, Suite 300. 6000 Broken Sound Parkway NW, Suite 300.
- Spread Plate Technique: Principle, Procedure, Results • Microbe Online
- Difference Between Pour Plate and Spread Plate | Compare the Difference Between Similar Terms
- BAM Chapter 3: Aerobic Plate Count | FDA
- Obtaining Pure Culture of Microorganisms: 6 Methods (biologydiscussion.com)
- Microbiology – 004 – Spread Plate Method | Microbiology Undergraduate Program (iastate.edu)
- Spread Plate Technique For Isolation of Microorganism | Culture Methods (paramedicsworld.com)
- Spread Plate Technique- Principle, Procedure and Uses (microbiologyinfo.com)
- Spread Plate Method: Procedure, Principle, Purpose, Result. (microbiologynote.com)

Please correct the point number 10 in precautions of spread plate, 2nd sentence as 24 hours after INCUBATION, not inoculation
Thanks for the correction. The page has been updated.
There is no observation
The others are very helpful
Is spread plating bacterial suspension of 0.01ml acceptable?
ES DE MUCHA IMPORTANCIA EL TEMA, GRACIAS POR PUBLICARLO.
I need to know more about microbiology can you help me, please?