Streak literally means “a long, thin line”: and the streak plate method is a microbiological culture technique where a sample is spread in a petri dish in the form of a long, thin line over the surface of solid media.
Interesting Science Videos
What is Streak Plate Method?
The streak plate method is a microbiological laboratory technique of isolating pure cultures, and/or getting well-isolated colonies of bacteria from a mixed population. It is mostly used to get pure cultures of bacteria; however, yeasts can also be isolated by this method. It is one of the most commonly used aseptic techniques in microbiology to isolate and propagate bacteria. It is a mechanical isolation technique used in microbiology, commonly known as the “streaking method”.
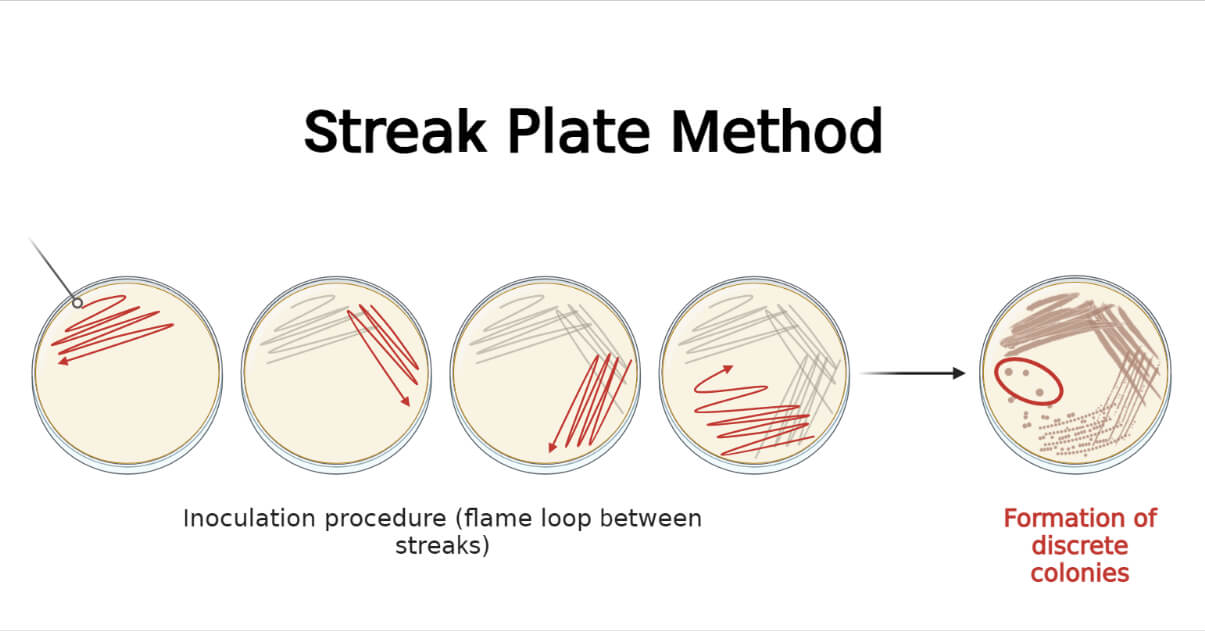
This method dilutes the bacterial load, over the surface of agar medium, successively as streaking proceeds, and ultimately only a few bacterial cells will be inoculated at the end giving well-isolated colonies in the final streaks. Thus, this method mechanically isolated the bacteria from a mixed population of either the same or different species. After inoculation, the same types of colonies are seen in the terminal streaks if the specimen contained single species, whereas, different types of colonies may be seen if the specimen contained different species.
This is a very old method used in microbiology since the time of Robert Koch. This method was first devised and used by Loeffler and Gaffky in Koch’s laboratory to serially dilute bacteria over agar surface and obtain well-isolated colonies. Since that time it is used as a very important tool in bacteriology.
It is a very simple and reliable aseptic technique that uses tools like cotton swabs, wooden or plastic, metal sticks and toothpicks, or inoculating loop to dilute and spread the specimen over the surface of pre-sterilized specific solid culture media. The specimen used can be either suspension or colonies from the agar surface. Well isolated colonies can be obtained from successfully performed streaking which allows describing the colony character of the organism on that specific culture media and condition.
Objectives of Streak Plate Method
- To obtain a pure culture of bacteria from a mixed culture
- To obtain well-isolated colonies
- To propagate bacteria
Principle of Streak Plate Method
The streak plate method is based on dilution during the process of mechanical spreading of inoculum over the surface of solidified culture media in order to obtain well-isolated colonies of the sample at the terminal streaks. Sample can be either colony on solid media or suspension in broth. The sample is picked by using different tools, mostly using a sterile inoculating loop or swab. The sample is placed over a surface of sterile solid media at one edge of the petri dish and a smear is prepared. Using the tool, the smear is successively streaked over the agar medium on different patterns. As the streaking proceeds, the inoculum is gradually diluted to the point where bacterial cells are separated as individual cells or as a colony-forming unit (CFU) at a gap of a few millimeters. When these inoculated plates are incubated, the isolated bacterium or a CFU will give rise to a well-isolated colony. This will allow us to get a pure culture as well as describe the colony morphology of the organism.
Types of Streak Plate Method
Based on the pattern of streaking, the streak plate method can be classified into 4 types, viz.: Quadrant Streaking, T-Streaking, Continuous Streaking, and Radiant Streaking.
1. Quadrant Streaking
It is the most commonly used and the most preferred method where four equal-sized sections of the agar plate are streaked. It is also referred to as the “four-quadrant streak” or “four sectors” or “four-way streak” method.
In this method, each plate is divided into four equal sectors and each adjacent sector is streaked sequentially. The sector which is streaked first is called the first sector or the first quadrant, and it has the highest concentration of inoculum. Gradually the second, third, and fourth quadrants will have diluted inoculum. By the time the fourth quadrant is streaked, the inoculum is highly diluted giving rise to isolated colonies following the incubation.
Mostly, a discontinuous fashion of streaking is followed where the loop is sterilized at the end of each quadrant prior to streaking over the next quadrant. However, if the bacterial load is too small (or highly diluted), continuous fashion can also be used. In the latter, the loop needs not be sterilized at the end of every quadrant.
Although being the most popular method, it limits us to use only one specimen per plate. If we try two or more specimens in a single 10 cm plate, this method is not suitable.
2. T-Streaking
It is another method of streaking where the agar Petri plate is divided into three sections and each section is streaked. Hence, this method is also known as the “three-sector streak” method.
The media is divided into three sections by drawing a letter “T” and each adjacent section is streaked sequentially. By the time the final section is being streaked, the inoculum is diluted to the point to give rise to isolated colonies following the incubation. Mostly discontinuous fashion of streaking is followed; however, a continuous fashion can also be used in the very dilute specimen.
As in quadrant streaking, it is difficult to culture two or more samples in a single 10 cm plate using this method.
3. Continuous Streaking
It is another commonly followed method where an inoculum is evenly distributed in a single continuous movement from starting point to the center of the plate. There is no need to divide the plate and sterilize the loop during the process. It is easy and quick; however, the problem is that we can use it only if the inoculum is either very diluted or we just have to propagate pure culture rather than isolate one.
We can divide the 10 cm Petri plate into different sections (mostly 2 to 6), and in each section, we can streak different specimens following this method. Hence, it is used in the clinical laboratory to culture urine, sputum, pus, etc. if multiple samples have arrived at a single time. This will allow us to save media and get maximum output using a minimum resource.
4. Radiant Streaking
It is another method of streaking where the inoculum is first streaked at one edge and spread in vertical lines above the edge. Finally, the vertical lines are cross streaked diagonally. This method is suitable to propagate pure culture, and also in the case of a dilute specimen.
There are other modified forms of streaking like:
5. Semi-quantitative Streaking
It is routinely followed in urine culture. It is a modified form of continuous streaking. In this method, a calibrated loop (usually a loop of 1 or 2μl) is used to streak a certain volume of the liquid specimen. A loopful of the specimen is streaked in a horizontal line in the middle of the Petri plate, and the specimen is spread all over the plate in a single continuous back and forth movement. This method allows us to approximately quantify the viable load (in a range, not an exact number) as well as get the pure culture in a single go.
6. Zigzag Streaking
It is another form of continuous streaking where a loopful of the specimen is streaked all over the plate in a zigzag pattern in a single continuous movement. It is commonly done to propagate the pure culture and culture them in large quantities.
Requirements of Streak Plate Method
- Streaking tool
This is a sterile tool used to streak the specimen over the surface of culture media. The tools used for streaking are cotton swabs, inoculating loop (both metal and plastic), toothpicks, and wooden or metal or plastic sticks/wires. The most commonly used one is inoculating loop (nichrome wire loop).
(In this whole article, we will talk about inoculating loop.)
- Sample culture
Sample bacteria may be in the form of suspension, liquid broth, or colonies over solid media. The sample is picked by using an inoculating loop and transferred over the surface of fresh culture media to perform streaking.
- Solid culture media
Specific culture media is used for the isolation and differentiation of suspected (or specific) bacteria. The culture medium is a solid agar medium that is pre-solidified before use.
- Bunsen burner and other laboratory facilities
A Bunsen burner is used to sterilize the loop and also to create a sterile zone around the flame. Besides, other chemicals, sterilizing materials, and laboratory apparatus are also required.
Procedure or Protocol of Streak Plate Method
The general procedure of the streak plate method can be summarized as:
- Arrange all the requirements, put on the PPE, sterilize the work surface, and allow all the samples and media to come to room temperature if were refrigerated.
- If the sample is very concentrated then dilution can be helpful to get the isolated colonies. (But it is not compulsory as the sample will be diluted during the streaking process.)
- Sterilize the inoculating loop by flaming and allow it to cool. Pick a small portion of the isolated colony. (if the sample is in the suspension then take a loopful of the sample)
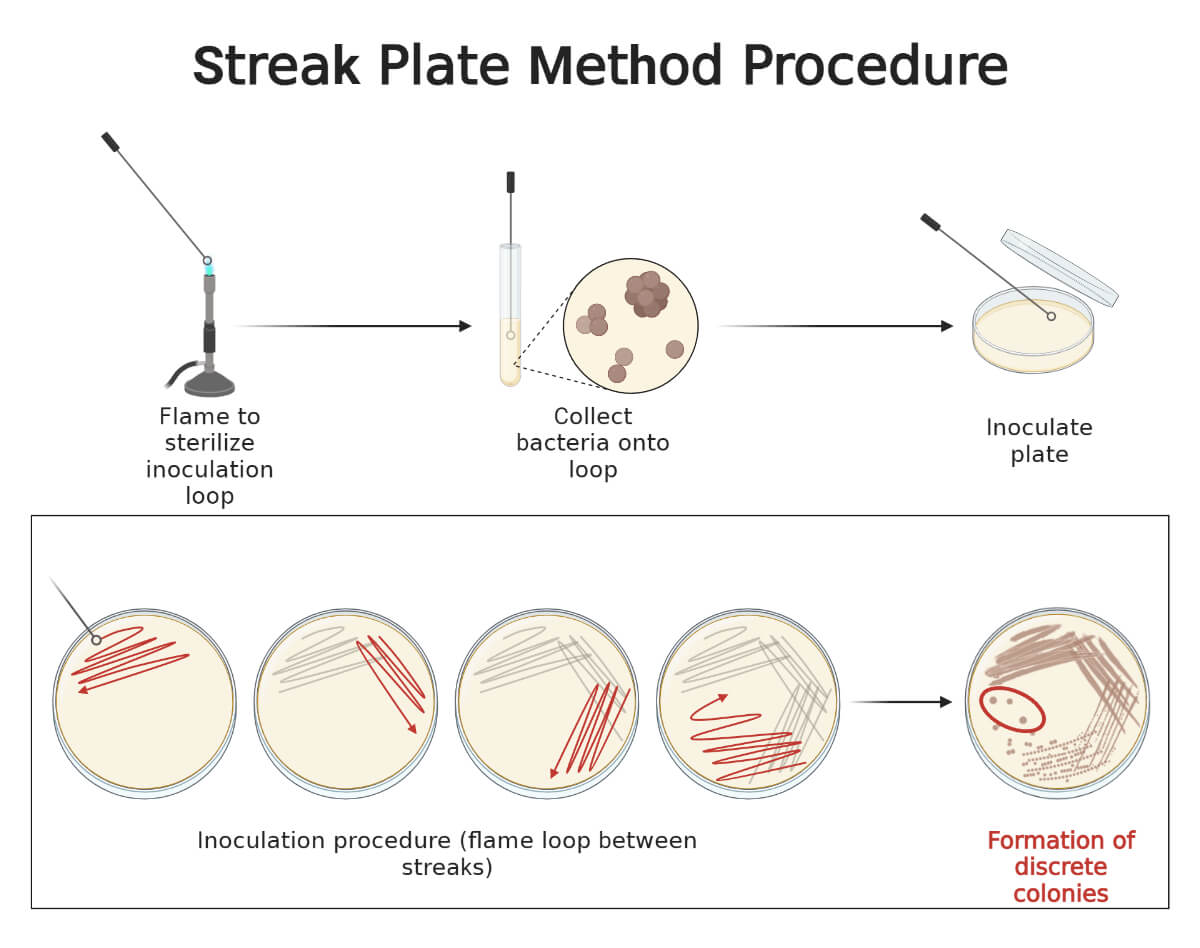
The inoculating procedure is different according to the method of streaking, let us deal with each type:
Quadrant Streaking Procedure
- Lift the Petri plate in your left hand and hold it at an angle of 60°.
(if you are left-handed, hold the plate in your right hand)
- The sample is spread over about 1/4th of the media in the Petri plate from the rim to the center of the plate using a rapid, gentle, back and forth motion.
(For ease, a beginner can draw two diameters intersecting each other diagonally at the back of the petri dish to divide the media into 4 equal sections)
- Re-flame the loop and allow it to cool. Turn the Petri plate by 90° anticlockwise, and place the loop to the last streaks of the first quadrant. Move the loop back and forth to spread the inoculum over the last half of the streaks in the first quadrant into the empty second quadrant.
(Be sure not to move the loop to the streaks in the first half of the first quadrant.)
- Repeat the process (iii) for streaking the third quadrant and the fourth quadrant.
- For the fourth quadrant similar step can be followed. However, many people prefer to draw a few (6 to 7 streaks) well-separated streaks by touching the second half of streaks in the third quadrant. Also, some prefer to make the final streak in a zigzag fashion making a tail.
(In a discontinuous fashion, the loop is sterilized after streaking each quadrant. In a continuous fashion, there is no need to flame the loop after streaking each quadrant. But, this is preferred only if the sample is very dilute.)
T-Streaking Procedure
- Lift the Petri plate in your left hand and hold it at an angle of 600. (if you are left-handed, hold the plate in your right hand)
- The sample is spread over about 1/3rd of the media in the Petri plate from the rim to the center of the plate using a rapid, gentle, back and forth motion.
(For ease, a beginner can draw a letter “T” at the back of the petri dish to divide the media into 3 sections)
- Re-flame the loop and allow it to cool. Turn the Petri plate by 900 anticlockwise, and place the loop to the last streaks of the first quadrant. Move the loop back and forth to spread the inoculum over the last half of the streaks in the first quadrant into the empty second quadrant.
(Be sure not to move the loop to the streaks in the first half of the first quadrant.)
- Repeat the process (iii) for streaking the third quadrant. As in quadrant streaking, you can follow any one of the streaking patterns at the 3rd quadrant.
Continuous Streaking Procedure
- Lift the Petri plate in your left hand and hold it at an angle of 600. (if you are left-handed, hold the plate in your right hand)
- Place the loop at one end of the plate and start streaking the inoculum from that point in a continuous movement to the center of the plate.
- Rotate the plate by 1800 and without sterilizing the loop, follow the step (ii) to streak the remaining half of the plate.
Radiant Streaking Procedure
- Lift the Petri plate in your left hand and hold it at an angle of 600. (if you are left-handed, hold the plate in your right hand)
- Spread the inoculum over the near edge of the agar plate using a gentle zigzag motion.
- Sterilize the loop and allow it to cool. Then, the streak from the point of primary spread in a radial direction up to the far edge of the Petri plate. (Be sure to streak the lines far away from each other.)
- Re-flame the loop and allow it to cool. Then draw horizontal lines crossing the radial streaks.
Semi-quantitative Streaking Procedure
- Lift the Petri plate in your left hand and hold it at an angle of 600. (if you are left-handed, hold the plate in your right hand)
- Using a calibrated loop take a loopful of the sample (urine).
- Draw the sample into a vertical or horizontal streak (primary streak) at the center of the plate.
- Using the same loop spread the inoculum by continuously moving the loop in back and forth (zigzag) motion crossing the primary streak.
- Label at the edge of the bottom of the plate with the date, name, sample ID, and other required information.
- Incubate the plate in an inverted position under suitable incubation conditions (mostly for 24 hours at 370C).
Result Interpretation of Streak Plate Method
- Results can be interpreted after the incubation period (mostly 24 hours at 370C). Following incubation, the terminal streaks are observed for isolated colonies. Morphologies of the isolated colonies are tabulated. If the culture media used is indicator type, then changes in the medium are also tabulated (E.g. lactose fermentation in MacConkey Agar).
- In the first area of streaking, there is heavy growth with fused colonies, and gradually there are fewer colonies in subsequent streaks giving a few well-isolated colonies in the final streak.
- Colonies with similar appearances are expected in pure culture.
- If the sample contained single species then colonies with similar morphologies are obtained. But, in the case of mixed culture, colonies with different morphologies are obtained.
- If there are different types of colonies, each colony must be streaked again in another plate to get a pure culture of each species.
[Exception: in some cases where colony characters of two or more bacterial species are the same, all the colonies may look alike even if they are of a different individual.]
Precautions during Streak Plate Method
- Media should be properly solidified before use. If it is refrigerated, allow it to come to room temperature.
- Check for the presence of water droplets and/or any contamination or foreign substance in media prior to streaking.
- Follow proper safety protocols. Treat every unknown or clinical specimen as hazardous and follow safety accordingly.
- If using a toothpick for streaking, use the blunt end by holding the pointed end between your thumb and ring finger at an angle of 10 to 200 to the medium. Dispose it after streaking each quadrant and take a new one to streak the second quadrant.
- If the sample is in suspension, properly mix the suspension before taking inoculum. Follow the aseptic technique during the process. Flame the rim of the test tube or bottle before and after taking the inoculum. If using a micropipette, don’t touch the wall of the tube or bottle with the pipette barrel.
- If the sample is a colony, gently touch the colony with a sterile and cool loop. Don’t take the entire colony or large portion, just touch the colony and it will be enough.
- Always work in a sterile area (between flames of a Bunsen burner or in a biosafety cabinet). Properly sterilize the inoculating loop before and after use. If flame sterilization is followed, make sure that the loop is cooled before using.
- Appropriate media should be selected.
- Use only a small amount of sample. Heavy inoculum doesn’t produce isolated colonies.
- Streak gently without applying high pressure.
- Flame the loop after streaking each quadrant.
- Rotate the plate anticlockwise after streaking each quadrant. Do not streak from the first half of the previous quadrant.
- Follow the suitable streaking pattern. If multiple samples are streaked in the same plate, ensure that there is at least a 20 – 30 mm gap between the streaking zones of each sample.
- Label properly and incubate under suitable conditions.
Applications of Streak Plate Method
- Used to obtain a pure culture from the mixed culture in order to perform morphological, biochemical, and molecular tests to identify and for other applications.
- Used to define the specimen as pure or mixed species.
- Used to study colony characters of bacteria.
- Used to produce a colony of genetically identical individuals
- Used in inoculation of clinical specimens in diagnostic laboratories to grow isolated colonies of pathogen
- Used in urine culture to isolate pathogens and semi-quantify the uropathogens to determine the significance of the infection. A calibrated loop is used for this purpose.
Advantages of Streak Plate Method
- It is a simple, reliable, convenient, and easy-to-perform method of inoculation.
- It results in well-isolated colonies, each of genetically identical individuals; hence, we can perform further tests and applications on the isolates. Hence, it is followed in clinical diagnosis.
- Dilution is done along with the process of inoculation (or streaking), hence, no need to perform separate dilution of the sample.
- Allow manually to control the sample and sample size and the inoculating area in a petri dish.
- Different patterns of streaking give flexibility in selecting the appropriate method based on sample size, availability of Petri dishes, and other requirements.
- It is a suitable and less-time consuming method to culture aerobic organisms.
- We can use a sample in both states; from the broth or suspension, as well as colonies from solid media.
Limitations of Streak Plate Method
- It is a qualitative isolation method, so don’t help in quantifying the microbial load.
- It is more suitable for aerobic organisms rather than anaerobes.
- Syntrophic bacteria can’t be purified by this method.
- It is unsuitable if the sample size is large and has a very high viable count. If we take heavy inoculum there may not be isolated colonies following the incubation.
- It needs specific media pre-solidified before the work. Hence, either we need prior information about probable microorganisms in the sample, or we have to have different types of media.
- There is a chance of tearing the agar surface during streaking if one is not skilled enough, and the media is freshly prepared.
- The method is time-consuming and requires an extra tool (inoculating loop) for streaking.
- There is a high chance of contamination during the process because we have to open the lid of the petri dish and constantly use the inoculating loop. Hence, there must be a sterile area and regular sterilization of the loop.
References
- Sanders E. R. (2012). Aseptic laboratory techniques: plating methods. Journal of visualized experiments : JoVE, (63), e3064. https://doi.org/10.3791/3064
- Textbook of Microbiology and Immunology (2012), 2nd Editions. Subah Chandra Parija. ISBN: 978-81-312-2810-4
- Practical Handbook of Microbiology, 2nd Edition.Edited by Emanuel Goldman and Lorrence H. Green. CRC Press. Taylor & Francis Group. 6000 Broken Sound Parkway NW, Suite 300. 6000 Broken Sound Parkway NW, Suite 300.
- VAN Soestbergen, A. A., & Lee, C. H. (1969). Pour plates or streak plates?. Applied microbiology, 18(6), 1092–1093. https://doi.org/10.1128/am.18.6.1092-1093.1969
- Streak Plate Method – Explained – Laboratoryinfo.com
- Streak Plate Method: Patterns, Procedure, Principle (microbiologynote.com)
- Streak Plate Method: Principle, Procedure, Uses • Microbe Online
- Microbiological Streaking Repair – iFixit
- Streak Plate Method: Principle, Purpose, Procedure, And Results – BIOCHEMINSIDER
- Streak Plate Method Principal and Types – RBR Life Science
- Streak Plate Method (Procedure) : Microbiology Virtual Lab I : Biotechnology and Biomedical Engineering : Amrita Vishwa Vidyapeetham Virtual Lab
- Streak plates (wisc.edu)
- Streak Plate – Virtual Interactive Bacteriology Laboratory (msu.edu)
- Streaking Agar Plates: 4 Quadrant Streak Method – Microbiology learning: The “why”ology of microbial testing (weebly.com)
- Streak Plate Technique For Isolation of Microorganism | Culture Methods (paramedicsworld.com)
- What is the purpose of streak plate method? (askinglot.com)
- Streaking method – Labster Theory

Thank you for detailed explanation
I searched online over and over for a detailed explanation on streak method without the resource leaving any part.
Thank you so much.
Exactly what I was looking for, thank you so much for this piece of information, it has met my expectations
Thanks for explaining. Streak Plate method testing means a long, thin line, and the streak plate method is a microbiological culture technique where a sample is spread in a petri dish in the form of a long, thin line over the surface of solid media. I like that you discuss the process streak plate method as a microbiological laboratory technique of isolating pure cultures and getting well-isolated colonies of bacteria from a mixed population. It is mainly used to obtain pure bacteria cultures; however, this method can also isolate yeasts. It is one of microbiology’s most commonly used aseptic techniques to isolate and propagate bacteria. It is a mechanical isolation technique in microbiology, widely known as the streaking method.
http://www.culturemediaconcepts.com/products/nonfat-dry-milk/
Thanks a lot your website is very useful for me I was able to make my own notes and easy to understand. Share pdf as well.
Please available all notes in pdf formet also so we can download.
Thanks
your website are very very usefull for me and for us