The pour Plate Method technique was established in the laboratory of Robert Koch and is still being used widely since his period. This method is suitable for facultative, Microaerophilic, and anaerobic microorganisms. It is simple, less resource-consuming, easy, and economical; however, it requires the sample to be in liquid or suspension.
Interesting Science Videos
What is Pour Plate Method?
The pour plate method is a microbiological laboratory technique for isolating and counting the viable microorganisms present in a liquid sample, which is added along with or before molten agar medium prior to its solidification.
This technique is generally used to count viable microorganisms in the given sample by enumerating the total number of colony-forming units (CFUs) within and/or on the surface of the solid medium. It is mostly used for enumerating bacteria; however, Actinobacteria, molds, and yeasts can also be isolated and enumerated.
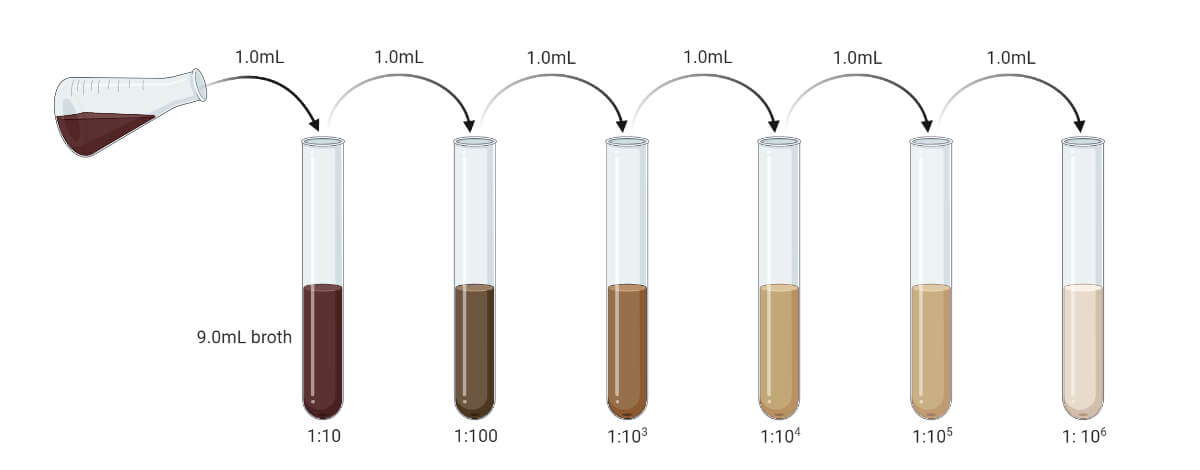
Prior to performing the pour plate technique, the sample must be serially diluted to make the microbial load in the sample between 20 – 300 CFU/mL (suitable colony counting range is 20 – 200, some consider it to 30 – 300, and in average it is taken as 25 – 250). If the sample is liquid, then it can be serially diluted with sterile distilled water or sterile broth. If the sample is solid or semisolid, it must be first emulsified and then serially diluted to reduce microbial load up to the permitted range.
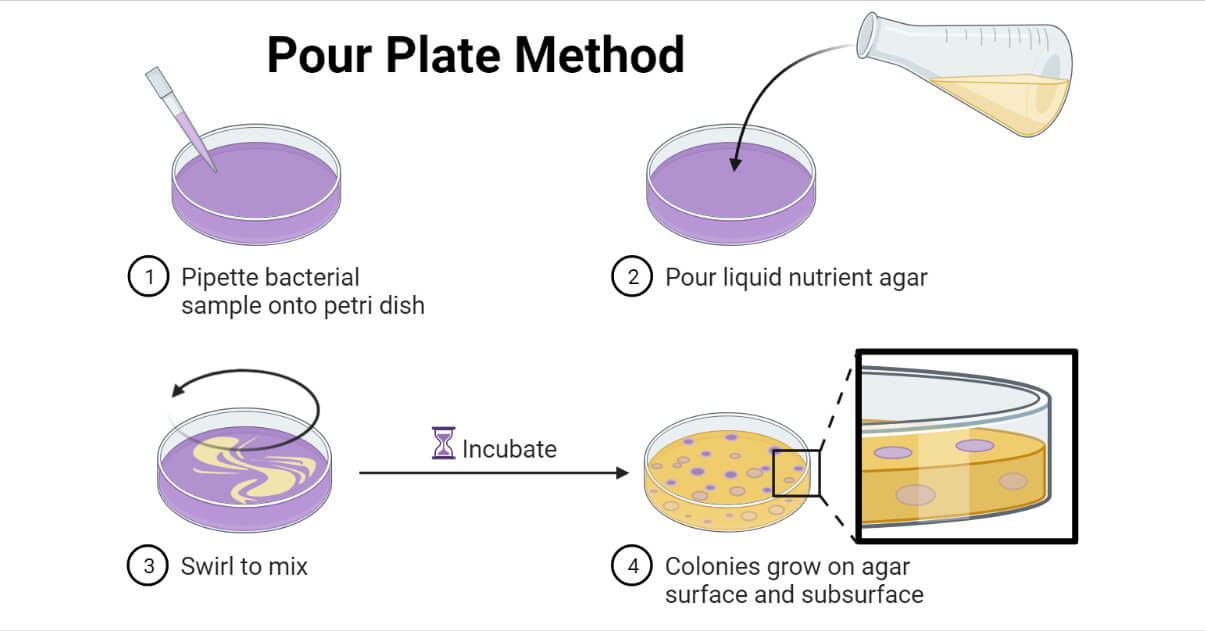
The sample is either added to the Petri plate and then the molten agar medium is poured over it, or the sample is mixed with the molten agar medium prior to pouring. After pouring in the Petri plate, the plate must be swirled quickly to properly mix the sample with the medium. The mixed medium is allowed to solidify and is incubated under the suitable condition to grow the microorganisms present in the sample.
Following the incubation, the numbers of isolated colonies are counted. If the colonies are uncountable or fused or more than 300 CFU/mL or less than 20 CFU/mL, it is recommended to repeat the process for getting the optimum count.
Objectives of Pour Plate Technique
- To isolate the microorganisms from the liquid specimen (or suspension)
- To calculate viable microbial load by counting colony formation unit (CFU) per mL
- To isolate the pure culture of microorganisms from a mixed population
- To isolate microorganisms in discrete colonies in order to study colony characters
Principle of Pour Plate Method
The pour Plate Method is based on the fact that when an agar medium mixed with microorganisms is incubated, each of the viable microorganisms will multiply forming a separate colony. In this method, a certain volume, usually 1 mL, of the serially diluted liquid sample is mixed properly with approximately 15 mL of specific molten agar medium of about 40 – 45°C (less than 50°C) in a Petri plate. The medium is allowed to solidify and is incubated, usually at 37°C for 24 – 48 hours. Following the incubation, the viable microorganisms in the sample will grow into visible colonies on the surface of and within the medium. The visible colonies can be counted and CFU/mL can be calculated using the following formula;
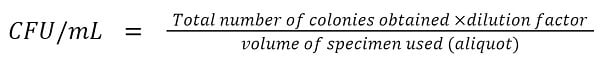
Requirements for Pour Plate Method
- Liquid Specimen (or suspension of the solid sample)
For the pour plate, the sample must be either liquid or in suspension form. The solid sample must be suspended in a suitable solvent that doesn’t influence the growth of any microorganisms or react with any material of the media.
- Suitable Solid Culture Media
Specific culture media is used for the isolation and differentiation of suspected (or specific) bacteria. The culture medium is a solid agar medium that is melted and is at a temperature of 40 – 45°C.
- Petri Plates
Mostly 10 cm Petri plates are used. They must be sterile and must be labeled before pouring samples or media.
- Test-tubes
Test tubes are needed for serial dilution during sample preparation. The tubes must be sterile.
- Sterile Distilled Water (or Sterile Broth)
Either distilled water or broth can be used for serial dilution. They are also used for dissolving the solid or semi-solid sample.
- Micropipette (or Graduated Pipette)
A micropipette of 0.1 mL or 1 mL capacity is required for measuring the sample during serial dilution and sample inoculation.
- Other Laboratory Facilities
Procedure of Pour Plate Method
The general procedure for performing the pour plate method can be summarized as follows:
1. Arrange all the requirements, put on the PPE, sterilize the work surface, and set up the laboratory types of equipment.
2. Sample preparation:
- If the sample is in liquid form, serially dilute it to make the microbial load to the range of 20 – 300 CFU/mL. (Prior pilot test may give exact value. You can prepare serial dilution up to 10-10 and use different dilutions.)
- If the sample is in solid or semisolid form, dissolve it in sterile distilled water or sterile broth, or any other solvent. (Generally, 1 gm sample is mixed with 9 ml of solvent to get the concentration of 10-1 gm/mL.)
3. Media preparation:
- Suitable media (general-purpose media like Nutrient Agar and Plate Count Agar for bacteria, and Potato Dextrose Agar or Sabouraud Dextrose Agar for fungi) are prepared and autoclaved. The media is allowed to cool to about 40 – 45°C (maximum up to 55°C), but don’t let it solidify.
- If the media is prepared already and solidified, melt it by placing it over a water bath or other heat source.
- If you want to mix the sample in media prior to pouring it into the Petri plate, you can either add approximately 15 mL of media in one test tube or beaker and autoclave it. Alternatively, a fixed volume of media can be prepared in a large beaker or bottle and a sample can be added later by calculating the volume which will be equivalent to 1 mL sample per about 15 mL of media.
4. Arrange sterile Petri plates. Label at the edge of the bottom of the plate with the dilution factor, date, name, sample ID, and other required information.
5. Inoculation:
Method – I
Dispense 1 ml of diluted sample in the center of the Petri plate using a sterile micropipette or calibrated pipette.
Open the lid of the bottle and flame its mouth. Pour about 15 mL of sterilized molten media at the appropriate temperature above the sample.
Close the lid of the plate then mix the sample in the media properly by gently swirling the plate. The plate is generally swirled in an “S” or “8” shape.
(Put sample at specific dilution in the plate labeled with the specific dilution factor.)
Method – II
In a tube with about 15 mL of molten media at a suitable temperature, add 1 mL of sample. Mix the sample properly in the media. Pour the media into a sterile Petri plate.
6. Close the lid of the Petri plate. Allow the media to completely solidify.
7. Incubate the plate in an inverted position under suitable incubation conditions (mostly for 24 hours at 37°C).
Result Interpretation of Pour Plate Method
- Following the incubation, developed colonies are observed. Each colony will account for one viable microbial cell or one colony-forming unit.
- If all the colonies are of the same type, then we can predict that the sample contained only one type of microbial genera. However, there may be different genera or species producing similar types of colonies. Hence, require a further test for their identification.
- If the colonies are of different morphology, then we can conclude that the sample contained a mixed population. They can be purified by sub-culturing each colony on a separate culture media plate using the streak plate method.
- Count the colonies and calculate CFU/mL by using the formula:
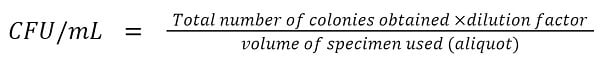
This will give the total number of viable microbial cells present in the given sample.
- For optimum count, the number of colonies must be between 20 – 300 CFU/mL. Beyond this limit, the whole procedure must be repeated. If the number of the colony is less than 20, it is suggested to use the sample of lower dilution, whereas, if the total number of colonies exceeds 300, it is suggested to use the sample of higher dilution on successive repeats.
- If the colonies are fused or the whole plate is covered with a single colony, then report as “too numerous to count” (TNTC) and repeat the process of taking the sample at a higher dilution.
Precautions during Pour Plate Technique
- Follow proper safety protocols. Treat every unknown or clinical specimen as hazardous and follow safety accordingly.
- Every Petri plate and media in use must be sterile. The working environment must be sterile.
- The water or media used in serial dilution or to dissolve the solid sample must be sterile.
- The solvent used to dissolve the solid sample must be sterile and must not have any growth-supportive or inhibitory effect against any microorganism.
- The sample must be diluted enough so that the viable microbial load falls between 20 – 300 CFU/mL. Above this range, it will be difficult to count the colony and the colonies may fuse together. Below this range, the result is reported as not significant. The process must be repeated under the same condition if the colony count is below 20 CFU/mL or above 300 CFU/mL.
- Accurate measurement is compulsory during serial dilution and media preparation.
- Use micropipette or standard calibrated tube while transferring sample in the Petri plate or in media tube or bottle.
- The permitted volume of media is 12 to 15 mL per 10 cm Petri plate by the US FDA, while the USP permits the volume of 15 to 20 mL per 10 cm Petri plate.
- The sample must be properly mixed with the molten agar before solidification.
- The temperature of media during pouring or mixing with the sample is very important. It must be between 40 – 450C (550C is the maximum temperature and must not be more than this at any cost). The temperature below 400C is not recommended because the media will either solidify or clumps may start developing.
- Each Petri plate must be labeled prior to pouring of media and sample. There must be labeling about dilution factor. While dispensing the sample, the labeling must be checked and the information about the dilution factor must be matched with the concentration of the sample.
Applications of Pour Plate Technique
- Used to isolate and enumerate viable bacteria and fungi (calculate CFU/ml) from suspensions or liquid samples.
- Used in food and pharmaceutical industries to isolate microorganisms and calculate CFUs from raw materials and products, like water, beverages, foodstuff, tissue samples, etc. This will help in quality control to ensure whether the product is safe to consume or not.
- Used to isolate and enumerate microorganisms from soil to study soil microflora.
- Used to generate growth curves while studying microbial metabolisms and biochemical features, and the effects of environmental factors on microbial growth.
- Used in getting discretely isolated colonies for obtaining pure culture and studying biochemical characters.
- Used in separating pure culture from mixed cultures.
Advantages of Pour Plate Technique
- It is easy to perform and doesn’t require extra tools or materials for inoculation.
- Don’t require previously solidified agar medium.
- Don’t have a risk of gouging during inoculation as like in streaking and spreading.
- Even a very low microbial load can be detected.
- Along with the isolation of microorganisms, their isolated colonies can be obtained. The number of CFU/mL can be calculated as well.
- It can use any type of specimen like clinical or environmental samples, liquid or solid (it can be dissolved).
- It is suitable for isolating facultative and anaerobic microorganisms. Aerobes can also be isolated by this method.
Limitations of Pour Plate Technique
- Solid or semisolid samples must be suspended prior to inoculation. It is very difficult if the sample is not easily soluble.
- There is a need for serial dilution of the sample otherwise too numerous colonies will be formed that can’t be counted or identified as discrete.
- Heat-sensitive organisms can be affected by molten media at 40-45°C.
- It requires some time for the growth of organisms and the formation of colonies. There will be less supply of oxygen to microbial cells at the bottom of the solidified media, hence growing slowly.
- Colonies may be smaller than in streaking or spreading which increases the chance of overlooking them.
- Obligate aerobes may face difficulty in growing at the bottom of the plates. Some don’t even grow.
- The method is time-consuming as it needs dissolving the solid samples, serial dilutions, and melting of the media at a certain temperature (42 – 45°C).
References
- Sanders E. R. (2012). Aseptic laboratory techniques: plating methods. Journal of visualized experiments : JoVE, (63), e3064. https://doi.org/10.3791/3064
- Textbook of Microbiology and Immunology (2012), 2nd Editions. Subah Chandra Parija. ISBN: 978-81-312-2810-4
- Practical Handbook of Microbiology, 2nd Edition.Edited by Emanuel Goldman and Lorrence H. Green. CRC Press. Taylor & Francis Group. 6000 Broken Sound Parkway NW, Suite 300. 6000 Broken Sound Parkway NW, Suite 300.
- VAN Soestbergen, A. A., & Lee, C. H. (1969). Pour plates or streak plates?. Applied microbiology, 18(6), 1092–1093. https://doi.org/10.1128/am.18.6.1092-1093.1969
- BAM Chapter 3: Aerobic Plate Count | FDA
- Pour Plate Technique For the Isolation of Microorganism | Culture Methods (paramedicsworld.com)
- Pour plate Method: Principle, Procedure, Uses And Limitations – BIOCHEMINSIDER
- Pour Plate Method Principle, Procedure, Objective, Result, Advantages (microbiologynote.com)
- Pour Plate Method Principle, Procedure, Objective, Result, Advantages (microbiologynote.com)
- Benson’s Microbiological Applications, Laboratory Manual in General Microbiology, 13th Edition. MC Graw Hill.
- Pour Plate Method: Procedure, Uses, (Dis) Advantages • Microbe Online
- 11 Pour Plate Method Best Practices – Microbiologics Blog
- Maturin, L. and Peeler, J. T. 2001. FDA Bacteriological Analytical Manual. Chapter 3, Aerobic Plate Count.
- United States Pharmacopeia. 2013. <61>, Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests
- MEDIA PREPARATION : THE PLATE POURING METHOD – Alliance Bio Expertise (alliance-bio-expertise.com)
- Obtaining Pure Culture of Microorganisms: 6 Methods (biologydiscussion.com)

I saw a live in Instagram now about this technique and I want to understand more about it. This explanation is amazing and helped me a lot!! Thanks for share knowledge!!
😊
Dear Sir
I have a question… I am working in Pharma company. We prepared Cefixime oral suspension..
sir plz direct me to prepare the sample solution for Pour plate method.
It’s very simple bro, just practice it
Gracias
Impressive