Single Cell Protein is the dried cells of microorganisms consumed as a protein supplement by humans or animals.
The protein is derived from cells of micro-organisms such as yeast, fungi, algae, and bacteria which are grown on various carbon sources for synthesis.
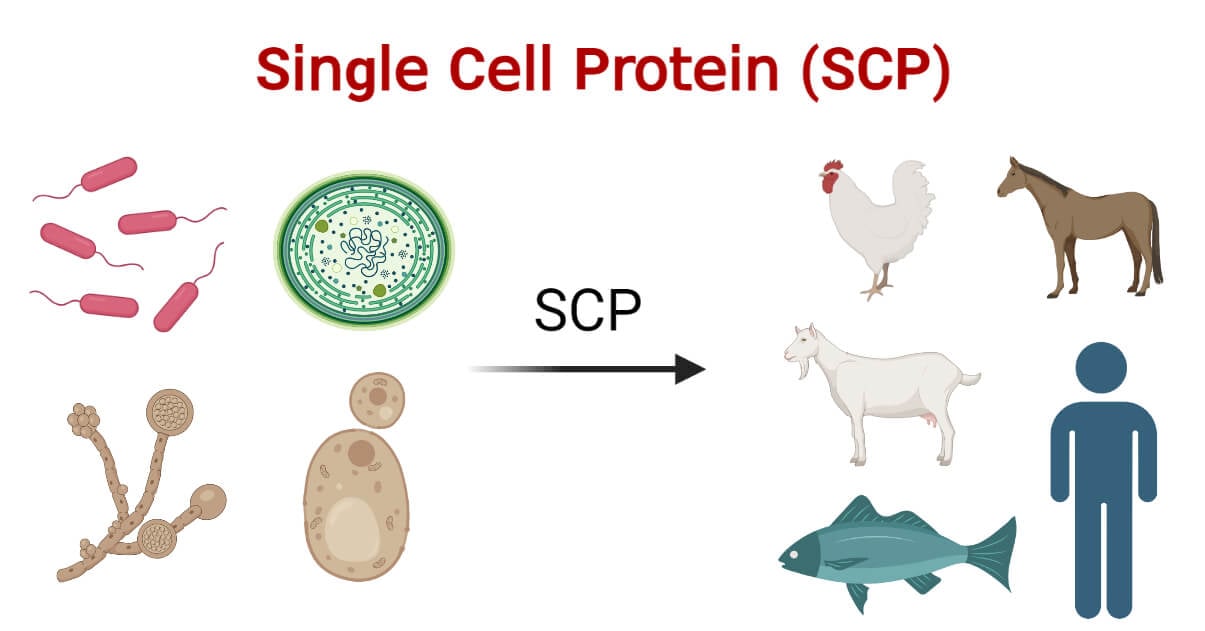
With the increase in population and worldwide nutrient deficiency, the use of microbial biomass as food and feed is highlighted.
SCPs contain vitamins, e.g., thiamine, riboflavin, pyridoxine, nicotinic acid, pantothenic acid, folic acid, biotin, cyanocobalamin, ascorbic acid, β-carotene and α- tocopherol; essential amino acids, represented by lysine and methionine; minerals; nucleic acids and lipids.
The microorganisms used in single-cell protein production should have the following properties.
- Absence of pathogenicity and toxicity
- Protein content and quality
- Digestibility and organoleptic qualities
- Growth rate
- Adaptability to unusual environmental conditions such as pH, temperature, and mineral concentrations.
- Ability to utilize carbon and nitrogen sources.
Interesting Science Videos
History of Single Cell Protein (SCP)
- The SCP was first developed during World War I.
- In 1919, Sak in Denmark and Hay duck in Germany invented a method named Zulaufverfahren in which sugar solution was fed to an aerated suspension of yeast instead of adding yeast to the diluted sugar solution.
- Saccharomyces cerevisiae was produced in Germany from molasses to replace protein.
- Candilaarborea and C. utilis were used during the Second World War.
- Similarly, during World War II Candida utilis was used in soup and sausage.
- Many industries were established in the USA and Europe, especially for C. utilis production after the war.
- During the 1960s and 1970s, production industries were established in the UK, France, Italy, Russia, Japan, and Taiwan.
- The term single-cell protein was coined by Carol L. Wilson in 1966.
- In the 1960s, researchers at British Petroleum developed a technology called the proteins-from-oil process for producing
- Initial research work was done by Alfred Champagnatar and BP s Lavera, Oil Refinery in France; a small pilot plant there started operations in March 1963.
- Champagnat was awarded with the UNESCO Science Prize in 1976.
- The Soviets were opening large “BVK” (belkovo-vitaminny kontsentrat, i.e., “protein-vitamin concentrate” plants next to their oil refineries in Kstovo (1973) and Kirishi (1974).
- The Soviet Ministry of Microbiological Industry had eight plants by 1989 but when the environmentalist movements began to give pressure on the government, they decided to close them down or convert to some other microbiological processes.
- In 1973, a few species of actinomycetes and filamentous fungi were reported for protein production by using various substrates, at Second International Conference which was held at MIT.
- In recent times, among the European Communist countries, Russia has had the greatest capacity for SCP production, with at least 86 plants in operation, using different substrates.
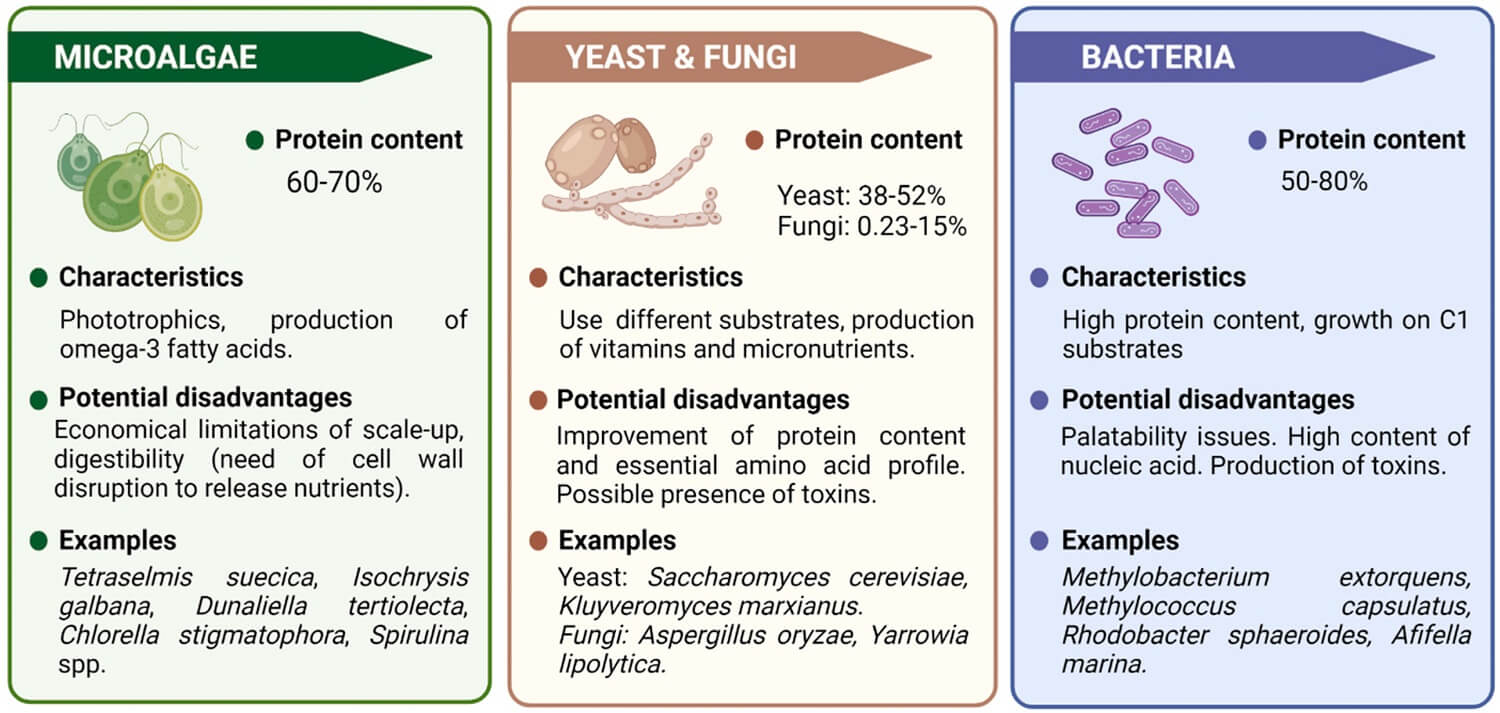
Organisms used as Single Cell Protein (SCP) and the substrate used for their production
| S.N. | Microalgae | Bacteria | Fungi | |
| 1 | They are phototrophic organisms. | They can be divided into unicellular yeast and mold. | ||
| 2 | They are potential SCP due to their chemical composition which contains proteins, essential fatty acids mainly omega-3 fatty acids, and several bioactive compounds. They have relatively low nucleic acid content (3–8%). | Bacteria are potential SCP as they possess high protein content (50-80%) along with vitamins, phospholipids, and other functional molecules. They are capable of growing on a wide range of substrates from carbohydrates to gaseous and liquid hydrocarbons | Yeasts are mainly used in aquaculture as it is the protein-rich ingredient in aquafeeds, with crude protein contents of 38–52%. | Mold is found to be highly digestible by fish. |
| 3 | The advantages of using algae include simple cultivation, effective utilization of solar energy, faster growth, and high protein and | The advantages of using bacteria are its use of a wide range of substrates, their short time for generation, production of vitamins and micronutrients. | The advantages of using yeasts include a high level of malic acid content, can grow in acidic pH and is easy to harvest. | The advantages of using mold include high nucleic content of up to 10%. |
| 4 | The potential disadvantage is the economical limitations of scale-up, digestibility (need for cell wall disruption to release nutrients), the large surface area needed for cultivation, and contamination risk in an open pond. | The potential disadvantages are palatability issues, high content of nucleic acid, and production of toxins. | The potential disadvantages include the possible presence of the toxin, slower growth rate, and lesser content of protein (45- 65%). | |
| 5 | Examples: Tetraselmis suecica, Isochrysis galbana, Dunaliella tertiolecta, Chlorella stigmatophora, Spirulina spp. | Examples: Mthylobacterium extorquens, Mrthylococcus capsulatus, Rhodobacter sphaeroides, and Afifella marina. | Examples: Yeast: Saccharomyces cerevisiae, kluyveromyces marxianus.Fungi: Aspergillus oryzae, Yarrowia lipolytica. | |
Fungi and substrates utilized by them and the protein content produced
| S.N. | Substrate | Fungi | Protein content |
| 1 | Maltose, Glucose | Aspergillus fumigatus, Rhizopus chinensis | |
| 2 | Cellulose, Hemicellulose | Aspergillus niger, A. oryzae, Cephalosporium eichhorniae, Chaetomium cellulolyticum | |
| 3 | Glucose, Lactose, Galactose | Penicillium cyclopium | |
| 4 | |||
| 5 | Hydrocarbons | Yarrowia lipolytica Candida tropicalis | |
| 6 | Ethanol | Candida utilis | |
| 7 | Methanol | Pichia spp.Pichia pastoris | |
| 8 | Cellulose, Pentose | Scytalidium aciduphilium, Thricoderma viridae, Thricoderma alba | |
| 9 | Glucose | Fusarium venenatum | 44 |
| 10 | Sulphite waste liquor | Paecilomyces varioti Candida utilis | |
| 11 | Starch, Glucose | Fusarium graminearum | |
| 12 | Starch | Saacharomycopsis fibuligera Candida utilis Saccharomyces cerevisiae | |
| 13 | Whey | ||
| 14 | Banana waste Apple pomace Citrus pulp Potato starch processing waste Waste liquor | Aspergillus niger | 18 17-20 25.6 38 50 |
| 15 | Orange peel | Aspergillus niger Rhizopus oryzae Aspergillus niger Saccharomyces cerevisiae | |
| 16 | Banana peel | Aspergillus terreus | |
| 17 | Bergamot fruit (citrus fruit) peel | Penicillium roqueforti, Penicillium camemberti | |
| 18 | Banana peel, pineapple peel, papaya peel | Phanerochaete chrysosporium, Panus tigrinus Phanerochaete chrysosporium | |
| 19 | Papaya waste, cucumber peelings, pomegranate fruit rind, pineapple fruit skin, and watermelon skin | Rhizopus oligosporus | |
| 20 | Orange peel | Trichoderma viride, Trichoderma reesei | |
| 21 | Orange pulp and brewer’s spent grain Dried potato and carrot skins Cucumber peels and orange peels Discarded foods (mix of fruits and vegetables) | S. cerevisiae | 38.5 49.3 53.4 39 |
| 22 | Cheese whey | Candida krusei | 48 |
| 23 | Whey and potato pulp | K. marxianus | 33.7 |
| 24 | Juice, pulp, and peel from oranges and lemons Corn stover effluent | R. opacus | 42-52.7 47-52.7 |
| 25 | Molasses Bagasse | Candida tropicalis | 56 31 |
| 26 | Rice bran | Cladosporium cladosporioides Penicillium citrinum Aspergillus flavus Aspergillus niger Aspergillus ochraceus Fusarium semitectum and sp1 and sp2 Monascus ruber | 10 10 10 11 10 10 9 |
| 27 | Wheat straw | Pleurotus florida | 63 |
| 28 | Lignin | Chrysonilia sitophi | 39 |
| 29 | Rice bran (deoiled) | Aspergillus oryzae | 24 |
| 30 | Cheesy whey filter | Trichoderma harzianum | 34 |
| 31 | Citrus pulp | Trichoderma virideae | 32 |
| 32 | Waste capsicum powder Poultry litter Potato starch industry waste Potato wastewater | Candida utilis | 29 48 46 49 |
| 33 | Spoiled date palm fruit | Hanseniaspora uvarum Zygosaccharomyces rouxi | 49 49 |
| 34 | Cheese whey Orange pulp, molasses, brewer’s spent grain, whey, potato pulp, malt spent | Kefir spp. | 54 23 |
| 35 | Brewery’s spent grains (hemicellulosic hydrolysate) | Debaryomyces hansenii | 32 |
| 36 | Liquid sucrose | Hansenula jadinii | |
| 37 | Cheese whey Orange pulp, molasses, brewer’s spent grain, whey, potato pulp | Kluyveromyces marxianus | 43 34 |
| 38 | Inulin, crude oil, glycerol waste hydrocarbons | Yarrowia lipolytica | 48-54 |
Algae and substrates utilized by them and the protein content produced
| S.N. | Substrate | Algae | Protein content |
| 1 | Anabaena cylindrica | 43-56 | |
| 2 | Aphanizomenon flosaquae | 62 | |
| 3 | Arthrospira maxima | 56-77 | |
| 4 | Chlorella ellipsoidea | 42.2 | |
| 5 | Chlorella ovalis | 10.97 | |
| 6 | Chlorella pyrenoidosa | 57 | |
| 7 | Chlorella spaerckii | 6.87 | |
| 8 | Chlorella vulgaris | 51-58 | |
| 9 | Dunaliella primolecta | 12.26 | |
| 10 | Dunaliella salina | 57 | |
| 11 | Dunaliella tertiolecta | 11.4 | |
| 12 | Porphyridium aerugeneum | 31.6 | |
| 13 | Porphyridium cruentum | 28-39 | |
| 14 | Scenedesmus almeriensis | 41.8 | |
| 15 | Scenedesmus obliquus | 50-55 | |
| 16 | Spirulina platensis | 60-71 | |
| 17 | Tetraselmis | 36 | |
| 18 | Tetraselmis chuii | 31-46.5 | |
| 19 | Soda ash effluent | Chaetomorpha antennina Ulva fasciata Chlorella | 14-18.2 13.7-18.6 |
| 20 | Tofu waste Tempeh waste Cheese waste | Chlorella spp. | 52.32 52 15.43 |
| 21 | Saline sewage effluents | Chlorella salina | 51 |
| 22 | Natural habitat | Gracilaria domingensis Gracilaria birdiae Laurencia filiformis Laurencia intricate Chondrus crispus Porphyra umbilicalis Gracilaria verrucosa | 6.2 7.1 18.3 4.6 20.1 15-37 7-23 |
| 23 | Wastewater | Chlorella sorokiniana Scenedesmus obliquus | 45 52 |
| 24 | Salinated water Desalinated water | Spirulina | 48.59 56.17 |
| 25 | CO2 and sunlight | Chlorella pyrenoidosa Scenedesmus quadricauda Spirulina maxima | 53 |
| 26 | n-Alkanes, kerosene | Candida intermedia, C. lipolytica, C. tropicalis, Nocardia spp | |
| 27 | Nannochloropsis spp. | 39.3 | |
| 28 | Nannochloropsis gaditana | 39.3 | |
| 29 | Desmodesmus spp | 37.3 | |
| 30 | Schizochytrium spp | 9.4-42.5 | |
| 31 | Chlorella vulgaris | 17.9 | |
| 32 | Scenedesmus spp | 48 |
Bacteria and substrates utilized by them and the protein content produced
| S.N. | Substrate | Bacteria | Protein content |
| 1 | Orange wastes, lemon wastes | Rhodococcus opacus | |
| 2 | Commercial shrimp feed | Afifella marina STW181 | >46 |
| 3 | Ram horn | Bacillus cereus Bacillus subtilis Escherichia coli | 68 71 66 |
| 4 | Potato starch processing waste | Bacillus licheniformis Bacillus pumilis | 38 46 |
| 5 | Soybean hull | Bacillus subtilis spp | 26 |
| 6 | Collagen meat packing waste in a fermenter | Bacillus megaterium | |
| 7 | Glucose, fructose | Corynebacterium ammoniagenes | 61 |
| 8 | Cellulose | Cellulomonas spp Alcaligenes spp | |
| 9 | Whey | Lactobacillus bulgaricus Candida krusei | |
| 10 | Synthetic growth medium | Cupriavidus necator Rhodopseudomonas spp. Rhodobacter capsulatus Rhodopseudomonas acidophila | 40-46 11 45 23 |
| 11 | Petrochemical wastewater | Haloarcula sp. IRU1 | 76 |
| 12 | Methane (natural gas) | Methylococcus capsulatus, Ralstonia sp., Brevibacillus agri | 67-73 |
| 13 | Gas and liquid products of sewage | Methylomonas and Methylophilus | <41 |
| 14 | Methanol | Methylophilus methylotrophus Methylomonas clara | 81 |
| 15 | Methane | Methylomonas spp. (Methanomonas) Methylococcus capsuiatus Trichoderma spp. | |
| 16 | Ethanol | Acinetobacter calcoaceticus | |
| 17 | Diesel oil in fermenter | Achromobacter delvacvate | |
| 18 | Supernatant and biogas | Methylophilus spp | 24 |
| 19 | Natural gas | Methilomonas.spp | 69.3 |
| 20 | Brewery wastewater | Rhizospheric diazotrophs | >55 |
| 21 | Wastewater from a latex rubber | Rhodopseudomonas blastica | 66.7 |
| 22 | Sludge and sago starch processing | Rhodopseudomonas palustris | 72-74 |
| 23 | Poultry slaughterhouse wastewater | Rhodocyclus gelatinosus | 67.6 |
| 24 | Pineapple waste | Rhodobacter sphaeroides P47 | 66.6 |
| 25 | Fermented pineapple extract | Rhodopseudomon as palustris P1 | 65 |
| 26 | Soyabean wastewater | Rhodobacter sphaeroides Z08 | 52 |
| 27 | Glutamate malate medium | Rhodovulvum sulphidophilum | 15.6 |
| 28 | Pig farm waste | Rhodocyclus gelatinosus | 50.6 |
| 29 | Sugar refinery wastewater | Rhodopseudomonas and R. fulvum | 58 |
| 30 | Miso-like effluent medium | Rhodocyclus gelatinosus | 63 |
| 31 | Dehydrated medium from pineapple peel waste | Rhodobacter sphaeroides P47 | 66.6 |
| 32 | Seafood processing wastewater | Rhodocyclus gelatinosus | 50 |
| 33 | Wastewater from noodle | Rhodopseudomonas palustris | 50 |
| 34 | Municipal wastewater | Rhodopseudomonas spp. CSK01 | 60.1 |
| 35 | Tuna condensate | Rhodocyclusg elatinosus R7 | 56 |
| 36 | Cassava waste | Rhodocyclus gelatinosus | 45 |
| 37 | Simulated wastewater | Rhodopseudomonas palustris | 45 |
| Photosythetic sludge | Rhodopseudomonas palustris | 74 |
Single Cell Protein production method
- Selection of substrate and strain
- The fast-growing microorganisms are selected that are rich in protein content and possess suitable growth characteristics.
- Then suitable substrates are chosen that are essential for the growth of selected microorganisms.
- Fermentation
- A fermenter is an instrument, which is set up to carry out the process of fermentation mainly the mass culture of plant or animal cells.
- The inoculated medium is then placed in a fermenter where the microorganisms grow and multiply. The conditions inside the fermenter, such as temperature, pH, and oxygen levels, are carefully controlled to optimize the growth of the microorganisms.
- Harvesting
- After fermentation, the microbial cells are harvested and separated from the growth medium.
- However, the harvesting and purification after production of SCP production remain a problem.
- Post-harvest treatment
- The harvested microbial cells are then dried to preserve them and reduce their volume, making them easier to store and transport.
- SCP processing for food
- The dried cells are then processed to remove impurities, improve their nutritional content, and enhance their flavor and texture.
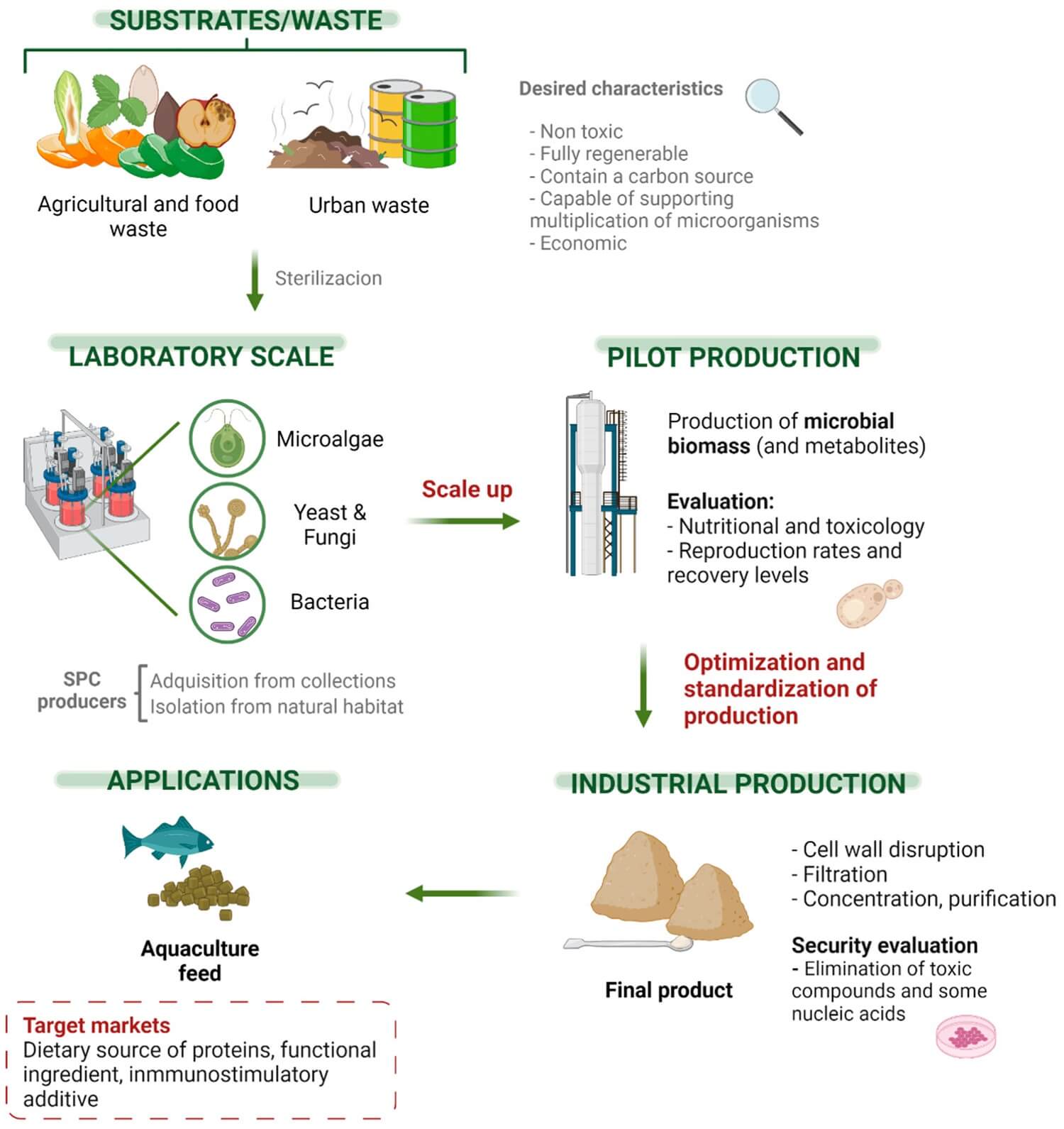
Advantages of Single Cell Protein
Microorganisms are usually used for the production of SCP because of the following advantages.
- Microorganisms grow at a faster rate compared to the growth of protein-rich grain which takes a year for production.
- The quality and quantity of protein are better (60-80%).
- A wide range of inexpensive raw materials can be used easily.
- The production process is easy and simple.
- The microorganisms can be easily subjected to genetic manipulation.
- The microorganism can be produced all-around a year.
- They can utilize a wide range of substrates.
- The production of SCP is eco-friendly, cost-effective, and energy efficient.
Disadvantages of Single Cell Protein
- The production of SCP is a complex process that requires strict control over various environmental factors.
- Maintaining the quality of SCP is a hard job as the harvesting and purification after production of SCP production remains a problem.
- SCP is not a suitable source of all the essential amino acids, so it is typically used as a supplement to other protein sources
- Despite the many potential benefits of SCP, consumer perception remains a significant challenge, as many people may be skeptical of consuming a product derived from microorganisms.
Applications of Single Cell Protein
- In animal feed and nutrition, for the stuffing and fattening of poultry, laying hens, calves, and pigs.
- As food additives (vitamin and aroma carriers and emulsifying agents), to enhance nutritional value (of baked food items, ready-made meals, soups, etc.), and as starter cultures (baker’s, brewer’s, and wine yeast).
- In industrial processes, as a foam-stabilizing agent, and in paper and leather processing.
- SCP provides the best protein-supplemented food for undernourished children as it serves as a good source of vitamins, amino acids, minerals, etc.
- They are used in therapeutic and natural medicines for controlling obesity, lowers the blood sugar level in diabetic patients, reduces body weight, cholesterol, and stress, and prevents the accumulation of cholesterol in the body.
- SCP is used in Cosmetics products for maintaining healthy hair, production of different herbal beauty products, like- Biolipstics, herbal face cream, etc.
References
- Abdullahi, N., Dandago, M. A., & Yunusa, A. K. (2021). Review on Production of Single-Cell Protein from Food Wastes. Turkish Journal of Agriculture – Food Science and Technology, 9(6), 968–974. https://doi.org/10.24925/TURJAF.V9I6.968-974.3758
- Ali, S., Mushtaq, J., Nazir, F., & Sarfraz, H. (2017). PRODUCTION AND PROCESSING OF SINGLE CELL PROTEIN (SCP)-A REVIEW. Retrieved January 26, 2023, from www.ejpmr.com
- Barka, A., & Blecker, C. (2016). Microalgae as a potential source of single-cell proteins. A review. Biotechnology, Agronomy, and Society and Environment, 20(3), 427–436. https://doi.org/10.25518/1780-4507.13132
- Bratosin, B. C., Darjan, S., & Vodnar, D. C. (2021). Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, Vol. 13, Page 9284, 13(16), 9284. https://doi.org/10.3390/SU13169284
- Gao, Y., Li, D., & Liu, Y. (2012). Production of single cell protein from soy molasses using Candida tropicalis. Annals of Microbiology, 62(3), 1165–1172. https://doi.org/10.1007/s13213-011-0356-9
- García-Garibay, M., Gómez-Ruiz, L., & Cruz-Guerrero, A. (2003). Yeasts and Bacteria. Encyclopedia of Food Sciences and Nutrition, 5277–5284.
- Kwatra, B., Yadav, P., Balasubramaniam, S., Garg, S., & Koyande, T. (2021). REVIEWING MAJOR MICROBES AS A SINGLE CELL PROTEIN. International Journal of Medical and Biomedical Studies, 5(3). https://doi.org/10.32553/ijmbs.v5i3.1842
- Nyyssölä, A., Suhonen, A., Ritala, A., & Oksman-Caldentey, K. M. (2022). The role of single cell protein in cellular agriculture. Current Opinion in Biotechnology, 75. https://doi.org/10.1016/J.COPBIO.2022.102686
- Pereira, A. G., Fraga-Corral, M., Garcia-Oliveira, P., Otero, P., Soria-Lopez, A., Cassani, L., Cao, H., Xiao, J., Prieto, M. A., & Simal-Gandara, J. (2022). Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods (Basel, Switzerland), 11(18). https://doi.org/10.3390/foods11182831
- Putri, D., Ulhidayati, A., Musthofa, I. A., & Wardani, A. K. (2018). Single cell protein production of Chlorella sp. using food processing waste as a cultivation medium. IOP Conference Series: Earth and Environmental Science, 131(1), 012052. https://doi.org/10.1088/1755-1315/131/1/012052
- Ritala, A., Häkkinen, S. T., Toivari, M., & Wiebe, M. G. (2017). Single cell protein-state-of-the-art, industrial landscape and patents 2001-2016. Frontiers in Microbiology, 8(OCT), 2009. https://doi.org/10.3389/FMICB.2017.02009/BIBTEX
- Sharif, M., Zafar, M. H., Aqib, A. I., Saeed, M., Farag, M. R., & Alagawany, M. (2021). Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture, 531(August 2020), 735885. https://doi.org/10.1016/j.aquaculture.2020.735885
- Simões, A. C. P., Fernandes, R. P., Barreto, M. S., Marques da Costa, G. B., de Godoy, M. G., Freire, D. M. G., & Pereira, N. (2022). Growth of Methylobacterium organophilum in Methanol for the Simultaneous Production of Single-Cell Protein and Metabolites of Interest. Food Technology and Biotechnology, 60(3), 338–349. https://doi.org/10.17113/ftb.60.03.22.7372
- Srividya, A. R., Vishnuvarthan, V. J., Murugappan, M., & Dahake, G. (2013). Single Cell Protein-A Review. International Journal for Pharmaceutical Research Scholars.
- Suman, G., Nupur, M., Anuradha, S., & Pradeep, B. (2015). Review Article Single Cell Protein Production: A Review. Int.J.Curr.Microbiol.App.Sci, 4(9), 251–262. http://www.ijcmas.com
- Thiviya, P., Gamage, A., Kapilan, R., Merah, O., & Madhujith, T. (2022). Single Cell Protein Production Using Different Fruit Waste: A Review. Separations, 9(7). https://doi.org/10.3390/separations9070178
- Vasey, R. B., & Powellf, K. A. (1984). Single-cell protein. Biotechnology and Genetic Engineering Reviews, 2(1), 285–311. https://doi.org/10.1080/02648725.1984.10647802

Is single cell protein nutritional as original protein?
nutritional
Very informative notes