What are Membrane Lipids?
Lipid molecules make up about 50% of the mass of most animal cell membranes. These are amphiphilic (amphipathic) molecules that are arranged into a two-dimensional sheet-like bilayer structure. Dual characteristics exhibited by the lipids in cell membranes can be attributed to the presence of a hydrophilic polar end and a hydrophobic nonpolar end in these molecules. The hydrophobic core formed by hydrophobic chains of lipids in each leaflet or layer is 3-4 nm thick in most biomembranes.
The three major classes of membrane lipid molecules are phospholipids, cholesterol, and sphingolipids. But, the most abundant membrane lipids are the phospholipids.
Interesting Science Videos
What are Phospholipids?
Phospholipids are compound/complex lipids containing lipid molecules attached to a phosphate group. Phospholipids are derivatives of glycerol-3-phosphate in which the glycerol backbone is attached to two fatty acids on one end and esterified phosphoric acid and an organic alcoholic group on the other.
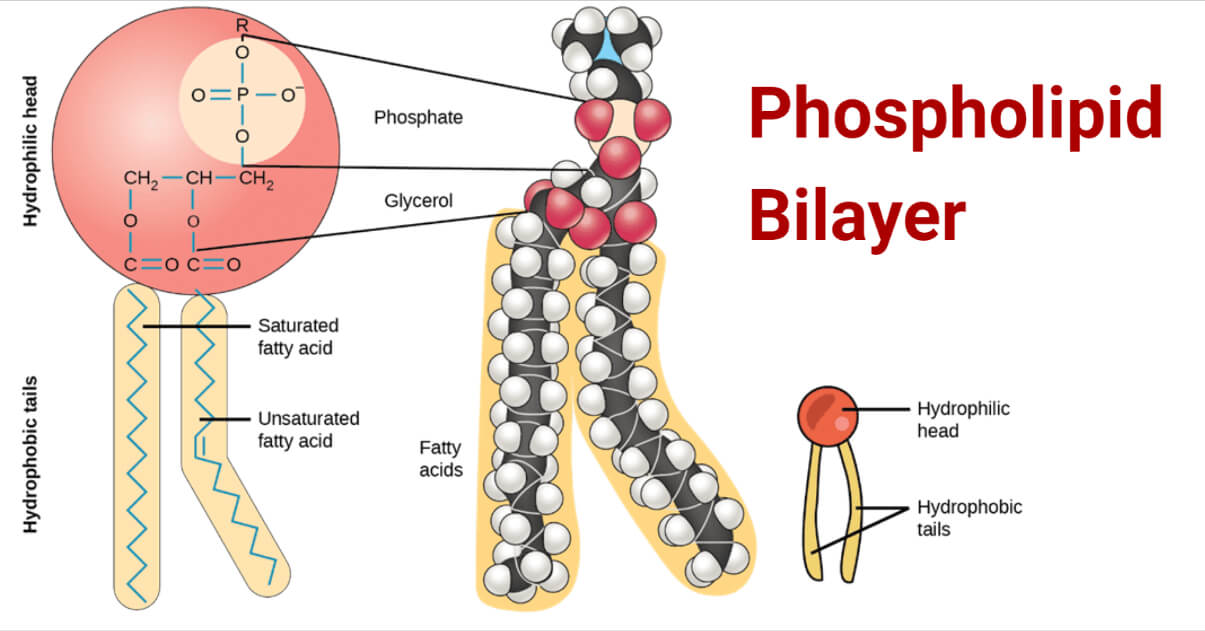
Structure of phospholipid bilayer
The phospholipid bilayer consists of phospholipids arranged in two layers with exterior facing hydrophilic polar heads and interior hydrophobic non-polar tails. This imparts the amphiphilic nature to phospholipids. Structurally, a phospholipid molecule comprises two fatty acid tails and a head with glycerol (3-carbon alcohol) and a phosphate molecule. The two fatty acyl chains are esterified to the two hydroxyl groups in glycerol, while the phosphate group is esterified to the terminal hydroxyl group in glycerol. The two fatty acyl chains differ in the number of C atoms (commonly 16 or 18) and their degree of saturation, i.e., the presence of 0, 1 or 2 double bonds. Surprisingly, the phospholipid fatty acids are of two types where one is saturated and the other unsaturated, which is responsible for membrane fluidity and flexibility of the phospholipid membrane.
Types of Phospholipids
Phospholipids are categorized into two types based on their backbone, namely:
1. Glycerophospholipids (phosphoglycerides)/ Glycerol phospholipids
Glycerol serves as the backbone. Depending upon the nature of its head group, different phosphoglycerides exist. They are:
- Phosphatidylcholine (PC): It is the most abundant phospholipid in the plasma membrane. Its head group has choline, a positively charged alcohol linked to the negatively charged phosphate group by ether bond(C-O-C).
- Phosphatidylserine (PS): Here, positively charged ethanolamine is attached to the negatively charged phosphate group by an ether linkage.
- Phosphatidylinositol (PI): Inositol is the head group.
The phosphate group, when bound to other head molecules such as hydrogen, and ethanolamine, it’s known as phosphatidic acid and phosphatidylethanolamine. Phosphatidic acid is considered to be the precursor to many phospholipids. Thus, it is the most fundamental one.
Plasmalogens are the members of phosphoglycerides that consist of one hydrocarbon chain attached to glycerol by an ester bond. In contrast, the other hydrocarbon chain is attached to glycerol by an ether linkage. These are highly abundant in the human heart and brain tissue.
2. Sphingophospholipids
Sphingosine act as the backbone. Sphingosine is amino alcohol with a long hydrocarbon chain. Sphingomyelins are phospholipids whose overall structure is quite similar to that of phosphatidylcholine. In sphingomyelin, phosphocholine is attached to the terminal hydroxyl group of the sphingosine backbone. It is a member of both phospholipids and sphingolipids.
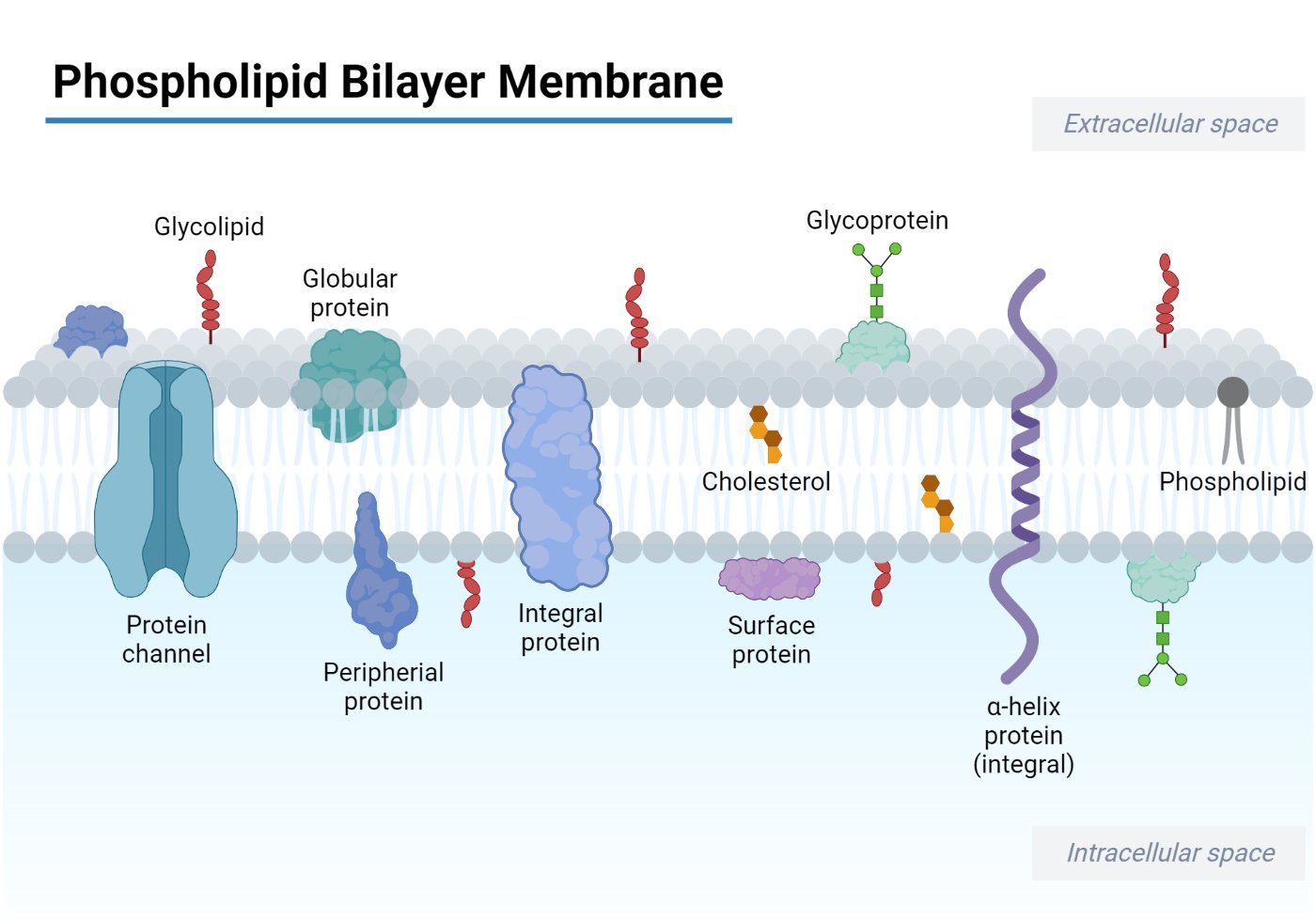
Arrangement in Membranes
Phospholipids are arranged in the bilayer structure with hydrophobic tails inside and hydrophilic heads outside the bilayer in an aqueous environment. The hydrophilic head molecule possesses charged or uncharged polar groups, which form electrostatic interactions or hydrogen bonds with water to make them readily dissolve in it. However, hydrophobic fatty acyl chains in the tail region are uncharged and non-polar, which doesn’t let them interact with water. To minimize the cost of free energy required to reorganize water molecules when phospholipids are dispersed in water, the phospholipids aggregate in such a way that they expose their hydrophilic heads to water and hide their hydrophobic tails in the interior. This results formation of spherical micelles or bimolecular sheets or bilayers.
Properties of the phospholipid bilayer
- Phospholipids are amphiphilic.
- Phospholipids can self-assemble. The major driving force for this property is the hydrophobic effect that repels the lipid chains from water to form micelles or liposomes.
- The hydrophobic core of the lipid bilayer prevents the diffusion of hydrophilic solutes across it. However, the presence of membrane proteins in the bilayer facilitates their transport across the impermeable barrier.
- The characteristic architecture of the lipid bilayer is stabilized by the hydrophobic and Van der Waals interactions between the lipid chains. However, the exterior aqueous environment varies greatly in its ionic strength and pH.
- Phospholipids in the lipid bilayer show either rotation or lateral movement in one bilayer, while transverse movement between bilayers in a “flip-flop” manner.
- Membrane fluidity is increased by phosphoglycerides while decreased by sphingolipids and cholesterol. The fluidity of the biomembranes is affected by several factors such as length and degree of saturation of fatty acids tails, temperature, and cholesterol content of the bilayer. The fluid nature of phospholipids allows several cellular processes, such as pinocytosis and endocytosis.
- Phospholipids are structural units of biological membranes and facilitate anchoring the membrane proteins.
- Phospholipids exhibit asymmetrical distribution in the two leaflets of the bilayer.
Functions of Phospholipids
Several biological functions are listed below:
- Phospholipids make up the structural component of most biological membranes, e.g., cell membranes, and these function to act as barriers to regulate the entry and exit of molecules to and from the cell.
- Some phospholipids, when degraded by an enzyme, produce products that act as second messengers in signal transduction. For example, the enzyme phospholipase C splits phosphatidylinositol (4,5)-bisphosphate into inositol triphosphate (IP3) and diacylglycerol (DAG). Both of these products have a potential role in signal transduction.
- Phospholipids participate in a wide range of cellular activities like apoptosis, phagocytosis, and regulation of mitochondrial physiology.
- Phospholipids contribute to cellular membrane fluidity and flexibility. Excessive alcohol intake decreases the number of phospholipids in hepatic cells, thereby reducing the flexibility of hepatic cells and leading to liver injury or cell damage. Thus, the administration of phospholipids is suggested to recover from this condition.
- Phospholipids are necessary for lipid metabolism and absorption.
References
- Alberts, B., Heald, R., Johnson, A., Morgan, D., Raff, M., Roberts, K., Walter, P., Wilson, J., & Hunt, T. (2022). Molecular Biology of the Cell (Seventh ed.). W. W. Norton & Company.
- Lodish, H., Berk, A., Kaiser, C. A., Krieger, M., Bretscher, A., Ploegh, H., Amon, A., & Martin, K. C. (2016). Molecular Cell Biology (Eighth ed.). W. H. Freeman.
- https://biologydictionary.net/lipid-bilayer/
- https://ibiologia.com/phospholipid-bilayer/
- https://www.biologyonline.com/dictionary/phospholipid
- https://chem.libretexts.org/Courses/Mount_Aloysius_College/CHEM_100%3A_General_Chemistry_(O’Connor)/17%3A_Lipids/17.04%3A_Membranes_and_Membrane_Lipids
- https://www.phospholipid-research-center.com/phospholipid/occurrence_and_benefits/
- https://www.mechanobio.info/what-is-the-plasma-membrane/how-do-lipid-bilayer-components-move/
- https://blog.cambridgecoaching.com/what-is-the-phospholipid-bilayer-and-what-determines-its-fluidity

Phospholipids exhibit symmetrical distribution in the two leaflets of the bilayer.
is this true???????????
Thanks for the correction. It should be “asymmetrical distribution”.
Dear Prakriti, We wish to use your first figure in a book chapter with due citations. please provide your consent.
Thanks and Regards
Vibha
Hi Vibha,
The original source of the first image is OpenEd CUNY. We have also given the source in the first figure.
Thank you.
Thanks
Am very grateful