Interesting Science Videos
Cholesterol Structure
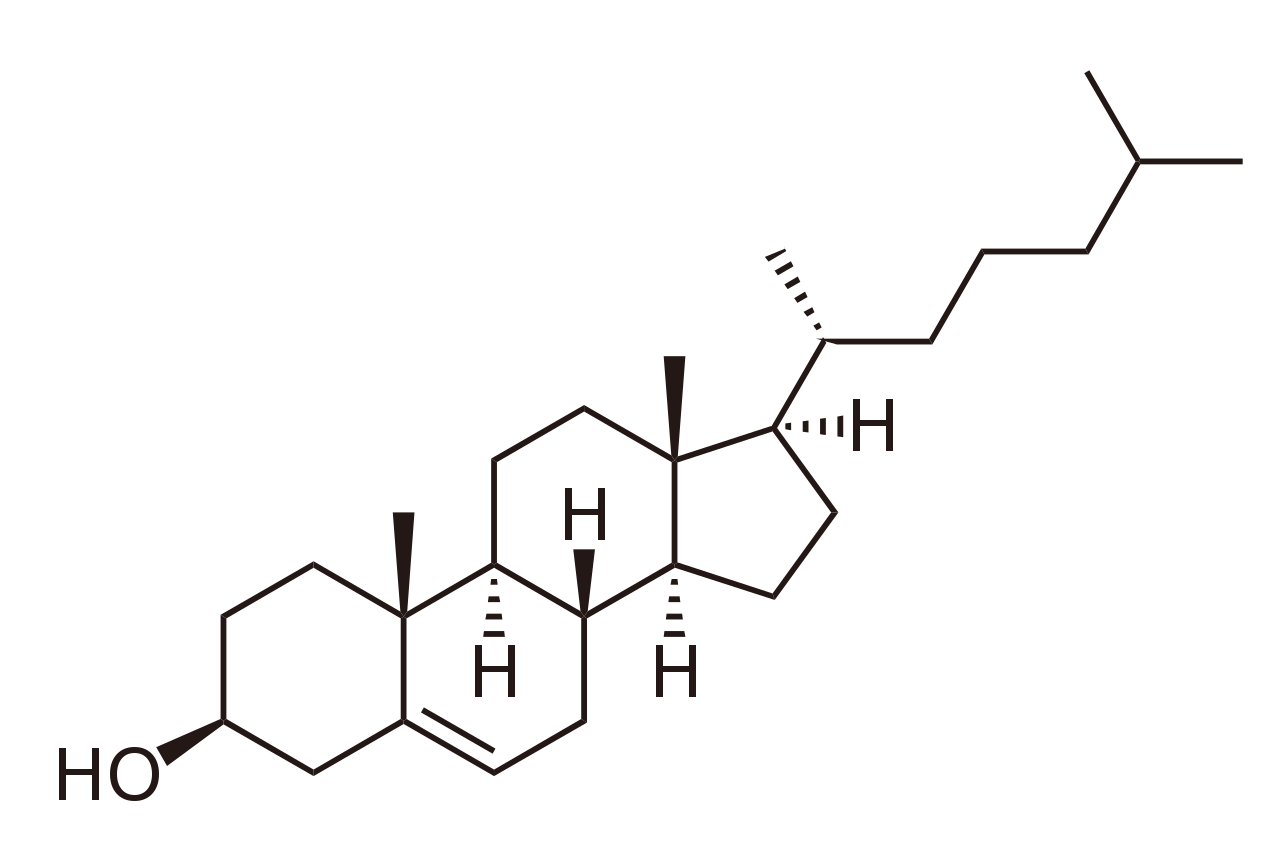
Figure: Cholesterol Structure
- Although cholesterol is synthesized in most tissues of the body where it serves as a component of cell membranes, it is produced mainly in the liver and intestine.
- Cholesterol and cholesterol esters are transported in blood lipoproteins.
- All the carbons of cholesterol are derived from acetyl-CoA.
- The key intermediates in cholesterol biosynthesis are HMG-CoA, mevalonic acid, isopentenyl pyrophosphate, and squalene. The major regulatory enzyme is HMG-CoA reductase.
Location of Cholesterol Synthesis
Cholesterol synthesis takes place in the liver and intestinal mucosa.
Substrates of Cholesterol Synthesis
- Acetyl CoA
- Acetoacetyl CoA.
Products of Cholesterol Synthesis
- Cholesterol: Oxidized to bile acids in liver; precursor for steroid hormones.
- Mevalonic acid: Precursor for terpenes (eg, vitamins A and K, coenzyme Q).
Cholesterol Synthesis Steps
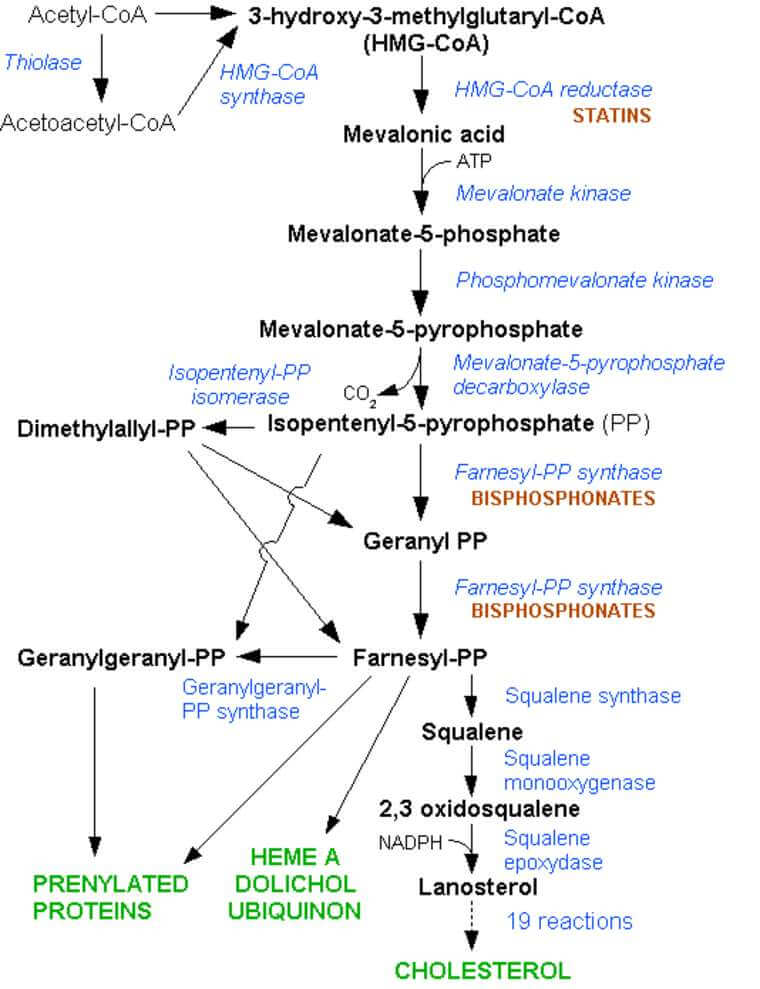
Cholesterol is synthesized from cytosolic acetyl-CoA by a sequence of reactions:
- Glucose is a major source of carbon for acetyl-CoA. Acetyl-CoA is produced from glucose by the same sequence of reactions used to produce cytosolic acetyl-CoA for fatty acid biosynthesis.
- Cytosolic acetyl-CoA forms acetoacetyl-CoA, which condenses with another acetyl-CoA to form HMG-CoA.
- Acetyl-CoA undergoes similar reactions in the mitochondrion, where HMG-CoA is used for ketone body synthesis.
- Cytosolic HMG-CoA, a key intermediate in cholesterol biosynthesis, is reduced in the endoplasmic reticulum to mevalonic acid by the regulatory enzyme HMG-CoA reductase.
- Mevalonic acid is phosphorylated and decarboxylated to form the 5-carbon (C-5) isoprenoid, isopentenyl pyrophosphate.
- Two isopentenyl pyrophosphate units condense, forming a C-10 compound, geranyl pyrophosphate, which reacts with another C-5 unit to form a C-15 compound, farnesyl pyrophosphate.
- Squalene is formed from two C-15 units and then oxidized and cyclized, forming lanosterol.
- Lanosterol is converted to cholesterol in a series of steps.
- The ring structure of cholesterol cannot be degraded in the body. The bile salts in the feces are the major form in which the steroid nucleus is excreted.
Regulation of Cholesterol Synthesis
- HMG CoA reductase is inhibited by high levels of cholesterol.
- In the liver, it is also inhibited by bile salts and is induced when blood insulin levels are elevated. It is also regulated by phosphorylation by the AMP-activated protein kinase.
- This enzyme is the pharmacologic target of lovastatin and other drugs in that class.
Circulation of Cholesterol Synthesis
Two-thirds of plasma cholesterol is esterified by LCAT, an enzyme activated by apo A. Cholesterol esterification by LCAT traps cholesterol in HDL and prevents membrane cholesterol uptake, which can lead to alterations in membrane permeability.
Significance of Cholesterol Synthesis
- Cholesterol is present in eukaryotic membranes and maintains membrane fluidity at a variety of temperatures.
- Cholesterol allows animal cells to function without a cell wall (which in other species protects membrane integrity and cell viability); this allows animal cells to change shape rapidly.
- Cholesterol is stored in tissues as cholesterol esters.
- In certain endocrine tissues, cholesterol is converted to steroid hormones.
- A cholesterol precursor can be converted to 1, 25-dihydroxycholecalciferol, the active form of vitamin D3.
References
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers.
- John W. Pelley, Edward F. Goljan (2011). Biochemistry. Third edition. Philadelphia: USA.
- Madigan, M. T., Martinko, J. M., Bender, K. S., Buckley, D. H., & Stahl, D. A. (2015). Brock biology of microorganisms (Fourteenth edition.). Boston: Pearson.
- Rodwell, V. W., Botham, K. M., Kennelly, P. J., Weil, P. A., & Bender, D. A. (2015). Harper’s illustrated biochemistry (30th ed.). New York, N.Y.: McGraw-Hill Education LLC.
