Lecithinase Test (Nagler’s Reaction) is a biochemical test for assessing bacteria’s ability to produce a toxic, phospholipase enzyme called ‘lecithinase’.
Lecithinase (Phospholipase C) is a lipolytic enzyme, produced by some bacteria, that hydrolyzes lecithin. Lecithin is a group of fatty acids (glycerophospholipids) and lipoproteins found in animal and plant tissues. Hence, lecithin is considered toxic and it has a great role in bacterial pathogenesis.
However, lecithinase is not produced by all bacterial species, even within a genus. This property of bacteria is taken advantage of to classify bacteria into two groups; lecithinase producers and non-producers. It is generally used to differentiate and identify species of food-born pathogenic bacterial genera like Clostridium species and Bacillus species.
Interesting Science Videos
Objectives of Lecithinase Test (Nagler’s Reaction)
- To assess the ability of bacteria to produce lecithinase enzyme
- To presumptively identify the bacteria
Principle of Lecithinase Test (Nagler’s Reaction)
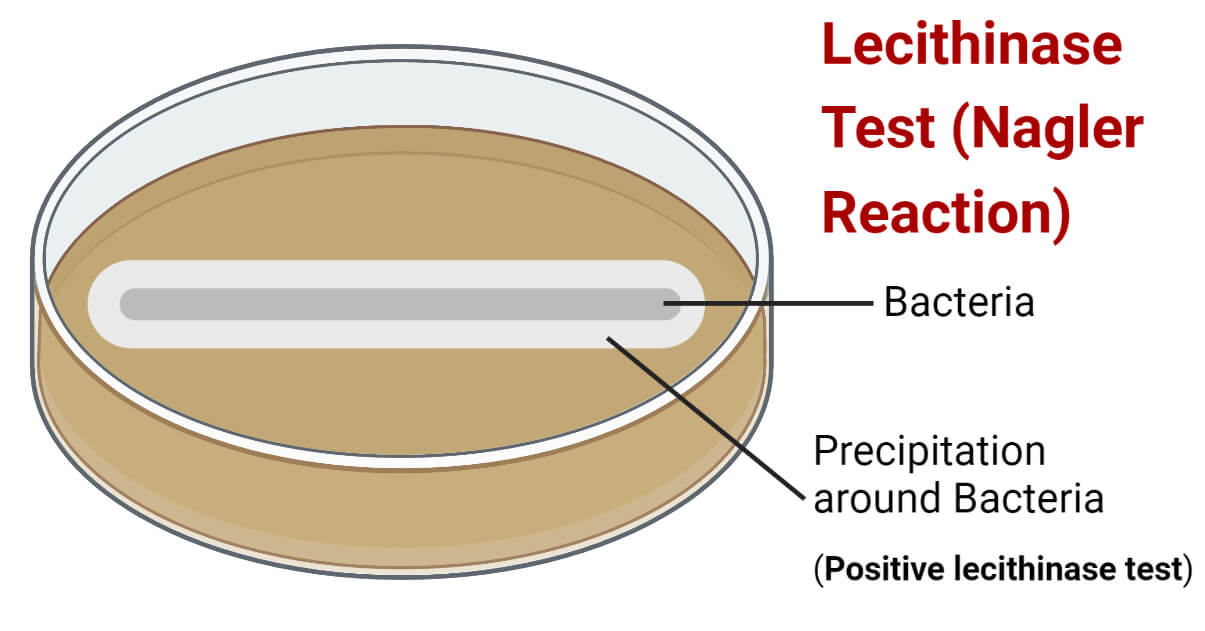
The egg yolk is a good source of lecithin, and it contains a significant amount of lecithovitellin. It is partly water soluble and is translucent, initially making the medium translucent. However, the lecithinase enzyme produced by bacteria will split lecithovitellin into phosphorylcholine and water-insoluble diglyceride. The diglyceride settles as a precipitate in the medium around the colonies of bacteria-producing lecithinase and develops an opaque white halo surrounding the colonies. This development of an opaque halo indicates hydrolysis of lecithovitellin and indicates a positive result.
Requirements for Lecithinase Test (Nagler’s Reaction)
a. Culture Medium
Agar medium containing lecithin is necessary for the detection of lecithinase enzyme production. Lecithin is a common component of an egg yolk; hence, Egg Yolk Agar medium is mostly preferred for the test. This medium is prepared by adding emulsified (50%) egg yolk to the sterilized Egg Yolk Agar Base medium.
Composition of Egg Yolk Agar Base per 1000 mL
Proteose peptone- 40.00 grams
Disodium hydrogen phosphate- 5.00 grams
Potassium dihydrogen phosphate- 1.00 grams
Glucose (Dextrose)- 2.00 grams
Sodium chloride- 2.00 grams
Magnesium sulfate- 0.10 grams
Hemin- 0.005 grams
Agar- 25.00 grams
Final pH 7.6 ±0.2 at 25°C
(Reference: Egg Yolk Agar Base (himedialabs.com))
Preparation of Egg Yolk Agar Base
- Measure the appropriate amount of Egg Yolk Agar Base powder (or the media components) and mix in the water of the required volume in a conical flask (or glass bottle) according to the instruction of the manufacturing company (75.10 grams of the above composition in 900 mL distilled water).
- Stir well using a magnetic stirrer or manually and heat to boiling so that all the components and agar dissolve completely in water.
- Autoclave the flask or bottle at 121°C and 15 lbs pressure for 15 minutes and let it cool to around 40 – 45°C.
Preparation of Egg Yolk Agar
- In the above-prepared base, dispense 100 mL of sterile egg yolk emulsion (egg yolk and distilled water in a 1:1 ratio) when the mixture is around 40-45°C.
- Stir the mixture completely so that the egg emulsion is properly mixed with the basal medium.
- Pour about 25 mL of the medium mixture into a 10 cm diameter sterile petri plate and let the medium solidify properly at room temperature.
b. Reagents
50% v/v egg yolk in sterile distilled water is required for making the Egg Yolk Agar medium.
It is always better to buy the readymade egg yolk emulsion because it is very difficult to prevent contamination during the process of making the emulsion. But, it can also be prepared in a lab following the steps listed below:
- Sterilize an antimicrobial-agent-free hen egg by rubbing it with spirit.
- In a sterile condition, scrub the egg and soak it in 95% ethanol for 1 hour.
- Break the egg within a sterile zone and aseptically separate the yolk.
- Dispense the yolk in a sterile beaker and add an equal volume of sterile distilled water.
- Stir properly to make a smooth egg yolk suspension.
c. Equipment
| Petri Plates Beaker | Weighing Machine Autoclave & Incubator | Bunsen burner Magnetic Stirrer | Inoculating loop |
PPE and other general laboratory materials
d. Test Organism (Sample Bacteria)
Positive Control: Clostridium perfringens ATCC 12924
Negative Control: Clostridium difficile ATCC 9689
Procedure of Lecithinase Test (Nagler’s Reaction)
- Using a sterile inoculating loop, pick up a heavy inoculum from a well-isolated colony of fresh culture (18 to 72 hours old culture).
- Inoculate the sample organism plate by drawing either a straight line or forming a circular inoculation of a size of a dime over the surface of the Egg Yolk Agar plate.
- Incubate the plates at 35±2°C for about 24 to 48 hours aerobically for aerobes or facultative, and for about 72 hours anaerobically for anaerobes.
(Incubation temperature varies according to bacterial species. Incubate according to the following for better results:
Bacillus spp., Gram-positive Rods and Anaerobes at 35°C
Non-glucose fermenting Gram-negative Rods (except P. aeruginosa) at 25°C
Glucose fermenting Gram-negative Rods including P. aeruginosa at 30°C)
- Following incubation, observe the development of a milky white halo around the bacterial colony.
Result and Interpretation of Lecithinase Test (Nagler’s Reaction)
A positive lecithinase test is indicated by the development of a milky white halo in the medium around the bacterial colony (line of growth).
A negative lecithinase test is indicated by no halo around the bacterial colony (line of growth).
Lecithinase Test Positive Bacteria
C. perfringens, Pseudomonas aeruginosa, (most of) Burkholderia spp., Bacillus cereus, B. thuringiensis, Bacillus anthracis, etc.
Lecithinase Test Negative Bacteria
C. sporogenes, C. butyricum, C. botulinum, C. difficile, Pseudomonas putida, Bacteroides fragilis, Bacillus subtilis, etc.
Quality Control
Clostridium perfringens ATCC 12924 produces a milky white halo around their colonies.
Clostridium difficile ATCC 9689 grows but does not produce any halo around their colonies.
An un-inoculated Egg Yolk Agar medium plate is necessary as a control plate to confirm a negative result.
Precautions
- As far as possible, use readymade egg yolk suspension. Making it in a lab is a high risk because it can be easily contaminated.
- Don’t add egg yolk emulsion to the medium base prior to autoclaving or when the medium is hot.
- Maintain the incubation temperature and oxygen/carbon dioxide level according to the sample organism
- The use of heavy inoculum is preferred for confluent growth and the development of a clear halo.
- An un-inoculated control plate should be incubated.
Applications of Lecithinase Test (Nagler’s Reaction)
- Identification and classification of Clostridium spp., and Bacillus spp.
- Identification of C. perfringens, S. aureus, Listeria monocytogenes, and P. aeruginosa.
Limitations of Lecithinase Test (Nagler’s Reaction)
- It is not a confirmatory test; hence, needs other biochemical test results for the complete identification of an unknown organism.
- Glucose non-fermenting rods produce a small halo zone and are difficult to read.
- Lecithinase is diffusible; hence it can diffuse all over the plate and makes the result interpretation difficult. A negative plate must be compared to the un-inoculated medium plate to confirm a negative result.
- Some may require a longer period of about 1 week for showing lecithinase activity.
- Some strains of P. fluorescens are positive while some are negative.
References
- Leber, Amy L., editor in chief. (2016). Clinical microbiology procedures handbook (Fourth edition). Washington, DC : ASM Press 1752 N St., N.W., [2016]
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier
- (2014). Lecithinase-producing bacteria in commercial and home-made foods: Evaluation of toxic properties and identification of potent producers. Journal of Taibah University for Science, 8(3), 207-215. https://doi.org/10.1016/j.jtusci.2014.03.006
- Matos JE, Harmon RJ, Langlois BE. Lecithinase reaction of Staphylococcus aureus strains of different origin on Baird-Parker medium. Lett Appl Microbiol. 1995 Nov;21(5):334-5. doi: 10.1111/j.1472-765x.1995.tb01073.x. PMID: 7576528.
- Lecithinase Test (Theory) : Microbiology Virtual Lab I : Biotechnology and Biomedical Engineering : Amrita Vishwa Vidyapeetham Virtual Lab
- Nagler’s reaction (Lecithinase test) | Medical Laboratories (medical-labs.net)
- Nagler Reaction: Principle & Procedure – BiochemGems
- Is the lecithinase test selective or differential? – ProfoundTips
- Nagler’s reaction – Oxford Reference
- Lecithinase Test – Principle, Procedure, Uses and Interpretation (microbiologyinfo.com)
- Nagler Reaction (Lecithinase Test) • Microbe Online
- Lecithinase Test: Result, Principle, Procedure, and Reagents (researchtweet.com)

Thank you for the information. I really needed it and i know it’ll come beneficial towards my lab experiments i am currently doing.