Electrophoresis is a chemical process in which an electric charge in a solution flow toward an opposing electrode. In the 1930s, Swedish biophysicist Arne Tisselius developed electrophoresis while researching blood proteins. In 1948, Arne Tisselius received Novel Prize in Chemistry for his contributions to the electrophoretic technique.
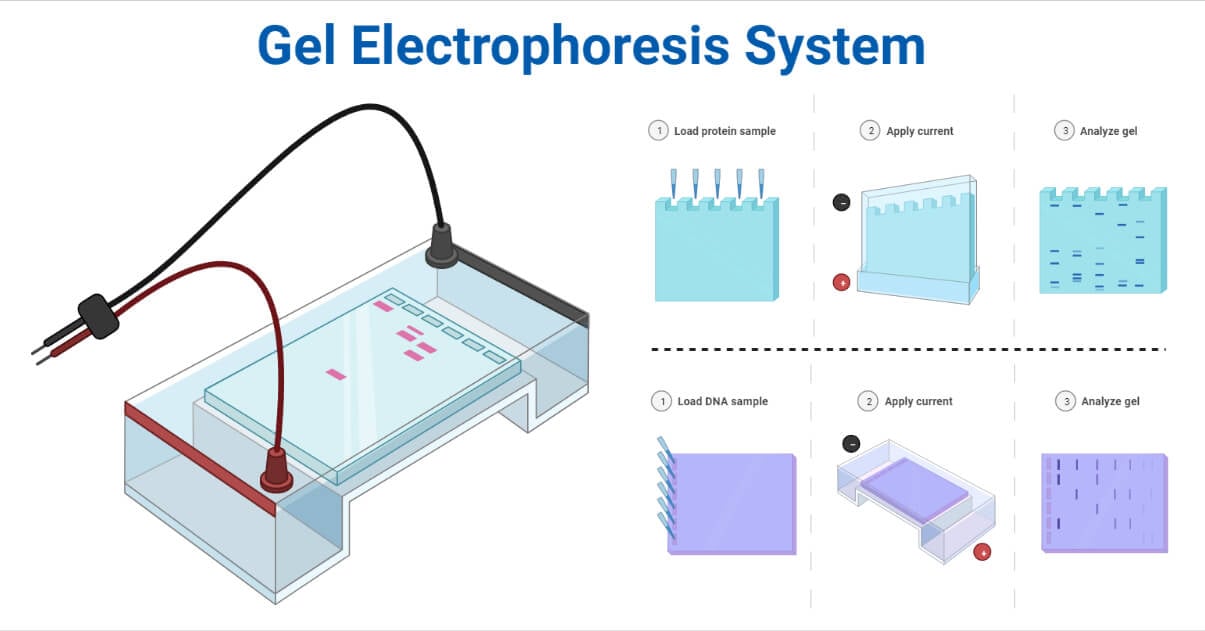
Gel electrophoresis is one of the laboratory methods for separating DNA, RNA, or protein molecules based on their electric charge or size.
Interesting Science Videos
Principle of Gel Electrophoresis
The principle behind electrophoresis is the observation that the majority of biomolecules exist as electrically charged particles with ionizable functional groups. A solution containing biomolecules will have either positively or negatively charged ions depending on the pH.
When charged molecules are placed in an electric field, they travel in the opposite direction of the positive or negative pole. Depending on the mass and net charge of each particle in the solution, ionized biomolecules will migrate at different rates when exposed to an electric field. Negatively charged particles such as nucleic acids gravitate toward the anode, while the positively charged particles toward the cathode. Each charged particle will migrate in a pattern determined by its particular property due to changes in speed and direction, allowing for the separation of biomolecule components with similar properties.
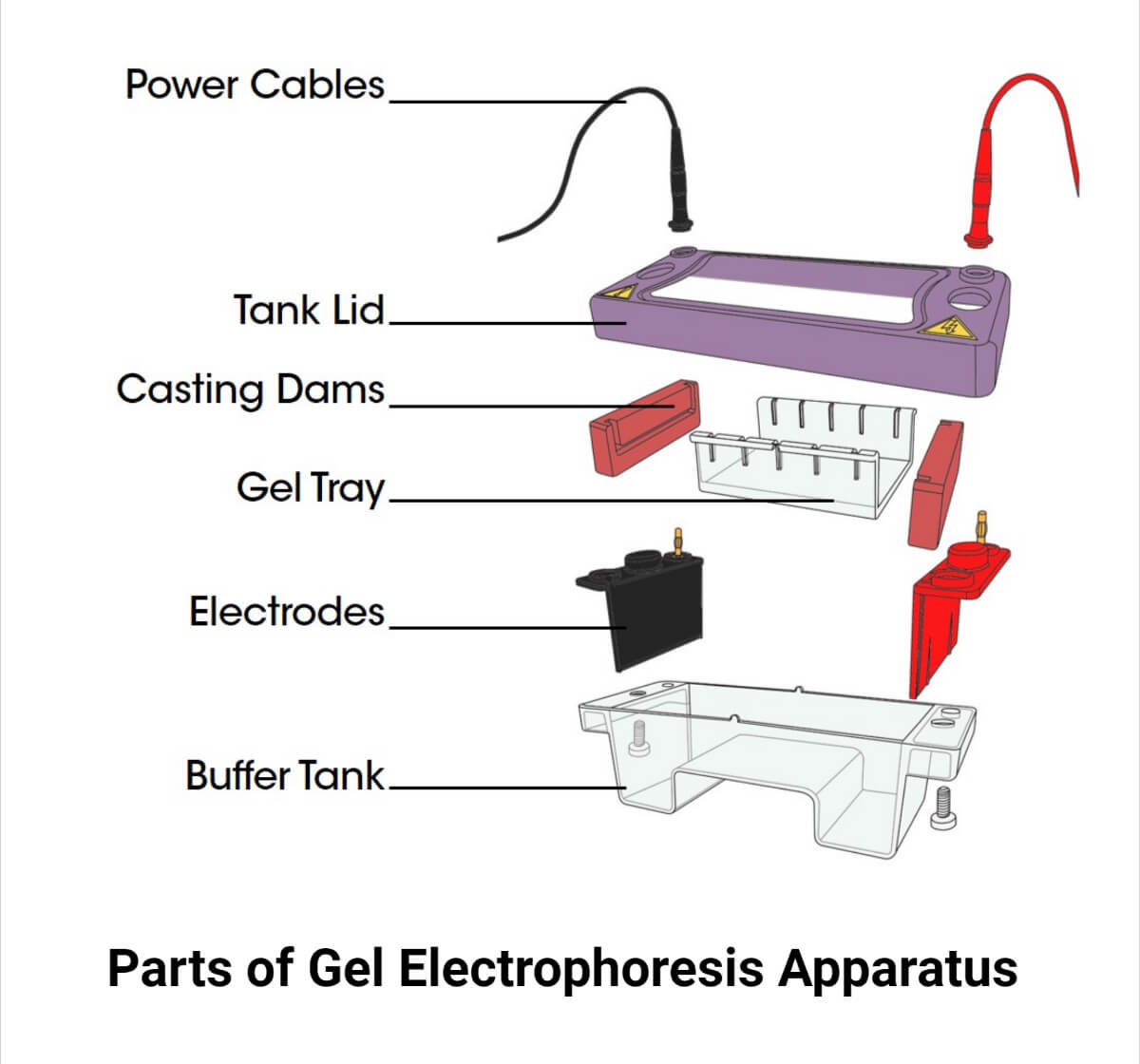
Parts of Gel Electrophoresis Apparatus
Power supply
- The conditions for electrophoresis are constant current, voltage, or power.
- A steady power supply should be used to maintain the migration pace.
- Lead cables with the colors red (anode/ positively charged electrode) and black (cathode/ negatively electrode) link the power supply to the gel box.
- These wires deliver the gel box with the electric current coming from the power source.
- If the current increases, more heat is produced through resistance, which causes the dissolved ions to stir thermally.
- Water from the equipment will evaporate more quickly.
- Ion concentration in the buffer will rise as a result of this.
- Because DNA and RNA are negatively charged, the black wire is attached to the rear of the box, which allows them to travel to the front of the gel box, where the positively charged red wire is attached.
Buffers
- The buffer establishes the pH of the system and the electrical charge on the solute.
- The ideal buffer has the following properties:
- Preserve the analyte’s ability to dissolve
- Keep the buffering capacity constant throughout the analysis
- It shouldn’t prevent the intended analytes from being detected.
- Achieve the appropriate range of separation
Two types of buffers exist Acidic buffer and a Basic buffer. For a lower pH, acidic buffers, including citrate, acetate, formate, and phosphate, are utilized. Basic buffers like tric, borate, and tricine are employed to keep pH levels high.
- The valency (ionic strength) and molality of the buffers are equal. Hence they are composed of monovalent ions.
- The prepared buffers should be carefully chilled while not in use since they can act as a favorable environment for the growth of bacteria.
- It is possible to use the cold buffer in the procedure since it increases sample resolution and reduces solvent evaporation.
- The buffer can be reused in large volumes up to four times, but in lesser volumes, it can be thrown away right away.
- Although there is a high risk of damaging heat-labile chemicals due to the high heat created, the higher ionic strength of the buffer is advantageous in obtaining a sharper resolution.
Support Media
- Supporting media include starch, polyacrylamide, agarose, and the membrane made of cellulose acetate in the form of sheets, slabs, and columns.
- It is a colloid that contains more than 90% water.
- It serves as a molecular sieve through which molecules are separated.
- Small molecules can pass through it because it is porous, while larger molecules cannot.
- Electrical neutrality is required.
- Agarose gel is now mostly employed as a support medium while conducting electrophoresis.
- Starch Gel
- It is the first gel medium used for electrophoresis.
- It facilitates the separation of proteins based on charge-to-mass ratio and molecular size.
- A colloidal suspension was prepared by boiling the suspension of starch granules in a buffer when allowed to cool sets as a semisolid gel due to the intertwining of the branched chains of amylopectin.
- Petroleum jelly is added to avoid swelling and shrinking.
- Sharp zones and high resolving power can be achieved.
- As gel preparation with reproducibility is challenging, it is not currently used.
- Cellulose Acetate
- When Kohn showed how to separate the protein hemoglobin found in red blood cells and to spot aberrant hemoglobin in blood serum, cellulose acetate electrophoresis was first developed.
- Filter papers, made entirely of cellulose, are acetylated to produce cellulose acetate. The glucose ring’s C-3 and C-6 locations are typically where acetylation occurs. Compared to other common electrophoretic matrices like agarose and polyacrylamide, cellulose acetate has bigger pores.
- Agarose
- Agar isolated from red seaweeds contains agarose, a naturally occurring linear polymer composed of galactose and 3,6- anhydro-galactose chains.
- Like agar, agarose is kept as a dry powder in storage.
- Agarose gel is cast by dissolving the agarose powder in the appropriate solution buffer, heating it, and letting it cool to room temperature.
- The agarose concentration in the solution buffer controls the pore size of the gel.
- To distinguish between DNA and RNA molecules, agarose gel is frequently used at 0.8% (W/V) to 5% (W/V).
- Relatively poor resolution compared to polyacrylamide gels.
- It has a low gelling temperature, a neutral charge, and forms stable gels. Thus, it is considered to be the perfect material for gel electrophoresis. It can either be solid or liquid.
- Polyacrylamide
- It is a clear, transparent gel formed by the copolymerization of acryl amide monomers in the presence of the crosslinking agent N, N- methylene- bis-acrylamide (also known as “bis-acrylamide”).
- Acrylamide concentration, which must be in proportion to its crosslinking agent, controls the size of the pores in polyacrylamide gels.
- Separating DNA and proteins typically requires a small amount of acrylamide gel (3%-15%).
- In sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (SDS-PAGE), proteins are separated under denatured conditions according to their size, where a higher percentage of acrylamide gel (10%-20%) is typically used.
Electrophoresis chamber
- It is a plastic container or tank filled with a buffer to prevent biomolecule movement.
- Its transparent lid makes it simple to see the migration process.
- It is wired to a power supply.
Container for staining and de-staining gel
- Gel staining and de-staining can be accomplished using trays and containers.
- There are lidded boxes and open-form boxes available.
- They typically have a propylene base.
- They are transparent and closely fitted.
- They are resistant to chemicals and stains.
Electrodes
- The two platinum electrodes help separate molecules due to their ability to attract charges with opposite charges.
- Positive ions are bound by an anode, while a cathode binds negative ions.
Gel Caster and Comb
- The gel is poured into a gel caster, which contains the gel and is stored inside the apparatus once it has dissolved in the solvent.
- The wells are placed using a comb to prepare them for sample loading.
Types of Electrophoresis
There are several types of gel electrophoresis, namely:
- Paper gel electrophoresis
- Agarose gel electrophoresis
- Polyacrylamide Gel Electrophoresis (PAGE)
- Pulse-field gel electrophoresis (PFGE)
- SDS- PAGE
- 2D- electrophoresis
- Immunoelectrophoresis (Rocket Electrophoresis)
- Difference Gel Electrophoresis (DIGE)
They are also categorized as native and denaturing, where RNA or proteins are kept in their native structure while running through the gel in native gel electrophoresis. In contrast, the RNA or protein are reduced to their linear structure before or during gel electrophoresis in denaturing gel electrophoresis. This reduction is achieved by the addition of a reducing agent to the sample, gel, and/ or buffer, which separates the bonds within the RNA or protein molecule and results in the formation of a secondary structure.
Paper gel electrophoresis
- It is used to investigate serum and other bodily fluids in clinical settings.
- Non-transparent and nontoxic
- Convenient to store
- Protein adsorption
- Poor conductivity
- Background staining
- Cellulose’s OH groups attach to proteins and slow electrophoretic motions, causing bands to the trail and the resolution to be poor.
Agarose gel electrophoresis
- The concentration of agarose determines the resolution of the electrophoresis.
- It is suited for separating DNA fragments ranging from 100 base pairs to 20 kilobase pairs.
- Additionally, it applies to the electrophoretic separation of proteins.
- When a low concentration of agarose gel is employed, it can be used to separate amphoteric molecules based on their isoelectric point, known as isoelectric focusing.
Polyacrylamide gel electrophoresis (PAGE)
- It is used at a concentration of up to 3-30% (pH range: 4-9.0): protein separation requires a higher concentration than DNA separation, and vice-versa.
- A greater degree of reliability and accurate porosity.
- Its application can be seen in calculating DNA’s molecular weight, DNA sequencing, studying DNA purity, analysis of recombinant DNA molecules and separation of RNA molecules, and measuring the molecular weight of RNA.
Pulsed-field gel electrophoresis (PFGE)
- In 1984, Shwartz and Cantor introduced this method.
- DNA separation in an agarose gel is accomplished by changing the direction and strength of the electrical field between electrodes.
- High molecular weight DNA with many megabases or even entire chromosomes are separated using this technique.
- PFGE is employed in many fields because it produces precise results that are efficiently reproducible.
- It is applied in the studies on the molecular biology of pathogens found in food, tracking the genetic stability of organisms employed in the fermentation process, mapping applications such as chromosome rearrangement detection, RFLP, and DNA fingerprinting, and identifying linked strains in the event of hospital outbreaks, etc.
Sodium dodecyl sulfate- Polyacrylamide gel electrophoresis (SDS-PAGE)
- Originally called the Laemmli Method after its British inventor U.K. Laemmli.
- Upper stacking gel has larger pores with a pH of 6.8, and Lower Separating Gel has smaller pores with a pH of 8.
- Proteins are separated based on polypeptide chain length in SDS-PAGE, which largely eliminates the influence of the structure and charge thanks to the use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel.
- SDS, a detergent in the sample buffer, and some reducing chemicals work together to damage the tertiary structure of proteins by rupturing their disulfide links.
- It is used to calculate the protein’s molecular weight and determine whether protein samples are pure or not.
Isoelectric point and Isoelectric focusing (IEF)
The pH level known as the isoelectric point is the one where proteins have no net charge (pI). Proteins are separated by their isoelectric points within a continuous pH gradient using the high-resolution approach known as isoelectric focusing (IEF). Compounds that differ in pI by only 0.01 pH units can be separated thanks to the excellent resolving power.
It is used to distinguish isoenzymes, fractionate proteins, and separate all amphoteric substances.
2D gel electrophoresis
- It is used to analyze complicated protein mixtures and was created as a hybrid of the 2DGel, IEF, and SDS-PAGE procedures.
- IEF separates the protein into its charges in the first step and later according to its mass in the second step.
- SDS treatment makes the separated protein on the IEF gel negatively charged, and the electrophoresis is carried out by placing the gel horizontally inside the SDS-PAGE gel.
- As a result, the proteins that are concentrated on the pI are divided based on their molecular weights.
Immuno-electrophoresis (Rocket Electrophoresis)
- In the process of immune-electrophoresis (IEP), firstly, electrophoresis is used to separate the protein antigen in semi-solid media, and then an immunodiffusion against the antiserum results in the creation of precipitin.
- Suitable antibodies complementary to the test antigen to be measured are dissolved in molten agar solution and placed on a horizontal plate. Antigens are injected into wells drilled in the gel. Ag picks up a negative charge at the alkaline pH, moves in the anode direction, interacts with Ab to form the Ag-Ab complex, ad precipitates. The immuno-precipitates will then appear as arcs like rockets once the gel has been stained with a suitable dye like CBB.
Difference Gel Electrophoresis (DIGE)
- It is created to address the quantitative element of differential-expression investigations and to alleviate some of the issues with 2D-PAGE, such as analytical fluctuations.
- To see each protein sample separately, up to three different protein samples can be tagged with fluorescent dyes that are size and charge-matched ( for example, Cy3, Cy5, Cy2). The three samples are combined, loaded, and subjected to 2D electrophoresis.
Operating procedures of Electrophoresis
Gel solution preparation: A gel is prepared by dissolving it in boiling water. After cooling to a more comfortable temperature, the solution is poured into a mold or caster.
Gel casting: A comb is used to create wells in the gel once it has been set. The gel is then inserted into the electrophoretic chamber. Buffer fills the chamber to a maximum of one-third of its total volume.
Sample preparation: To give the sample color and density, loading dye is added, which can be either a fluorescent tag or ethidium bromide.
The DNA is isolated and pre-processed, and placed in a solution with some basic blue dye to help visualize the movement of the sample through the gel.
Sample loading: A clean micropipette is used to load the sample into the wells.
Electrophoresis: The chamber and a power supply where the voltage is set are connected by the negative and positive leads, respectively. The electric field and negatively charged particles are created when the power supply is turned on. DNA that is negatively charged migrates toward the anode because molecules gravitate toward electrodes with opposing charges.
Stopping electrophoresis, Staining, and Visualization: Dye is used to following the migration visually. The power supply is turned off. The gel is stained and visualized using a gel imager when the procedure is finished. By comparing the size of the sample fragments to the standard, the logarithm of the molecular weight is used to calculate their sizes.
Applications of Electrophoresis
- DNA fingerprinting to separate DNA fragments to investigate crime scenes and paternity testing.
- Detection of genetic variations and proteins implicated in health and illness.
- It is employed in the detection and purification of nucleic acids and proteins for scientific purposes.
- It helps to find pathogens in the blood, other tissues, or sources like food.
- It facilitates the identification and purification of proteins or nucleic acids frequently examined in greater detail using mass spectrometry or DNA sequencing.
- It is used in blotting methods to analyze macromolecules and evolutionary studies.
- It facilitates the evaluation of results of Polymerase Chain Reaction (PCR).
- Vaccine development and manufacturing both benefit from electrophoresis.
- To differentiate species and evolutionary relationships, taxonomy-DNA profiling is performed.
Advantages of Electrophoresis
- Reasonably affordable.
- Establishes a direct connection between similar results
- Quite easy to carry out
- Can test DNA from any type of evidence.
- Superior resolution
- Available in a wide range of pore sizes.
- Stable over a wide range of pH, temperature, and ionic strength
- Transparent to light
- Chemically inert
- Electric neutrality and hydrophilicity
Limitations of Electrophoresis
1. Limited sample analysis
- Gene expression can be examined at each little location of a tissue sample using methods like in situ hybridization (ISH).
- With ISH, researchers may examine every brain region in a sample, whereas electrophoresis methods can only do so for a limited number of regions.
2. Measurements are not precise
- Gel electrophoresis can efficiently separate proteins with similar molecular weights using Western blotting.
- It can also separate proteins more precisely using a method called 2D electrophoresis.
- Mass spectroscopy must be used after the protein has been purified to determine the precise mass of proteins.
3. A substantial starting sample required
- Amplification of proteins is impracticable as done for DNA and RNA before electrophoresis. Thus, a sizable tissue sample is required to run these assays, which reduces the technique’s utility, and flow cytometry and immunohistochemistry are frequently used to analyze the protein expression in individual cells.
4. Limited visualization facility
- Electrophoresis is ineffective for measuring small hormones, neurotransmitters, and ions.
- Due to two issues, they don’t fully react to the electrophoresis preparation (commonly referred to as SDS-PAGE), and even if they did, they are too tiny to separate properly. They would rush out of the gel’s bottom.
5. Low throughput
- Low throughput in the sense that it doesn’t generate data very quickly. Compared to PCR and flow cytometry, which are massively parallel and serial processes, electrophoresis is inferior at producing research data and creating intricate relationships.
Precautions
- It is advised to use nonconducting floors and benches (made of wood or plastics).
- Avoid unintended grounding points and conductors (such as sinks and other waste sources) when operating around or close to an electrophoresis system.
- Avoid pushing hard while loading samples, as it may destroy wells.
- Put on gloves, face masks, and goggles while preparing gel.
- EtBr is carcinogenic, and mutagenic therefore take appropriate precautions before handling it.
Examples of Electrophoresis System
DNA electrophoresis system GEP-TH-1000TBT (Manufacturer: Bioevopeak)
Features
- The system features a built-in high-current power source that can quickly achieve high efficiency and rapid transfer by directly controlling the current between the titanium anode and the stainless-steel cathode.
- The transfer system seamlessly incorporates conventional transfer technologies, allowing for the quickest and most efficient protein transfer from gel to membrane.
Electrofocusing electrophoresis system BT105 (Manufacturer: G BIOSCIENCES)
Features
- Removes gel leak issues; no taping needed.
- There are two combs available for small and large wells.
- Supplied with a selectable power source.
- Easy to use and lightweight.
DNA electrophoresis system EPS-2014 (Manufacturer: INOVIALAB)
Features
- The EPS-2014 Mini Electrophoresis System is a compact, and clever design specifically for DNA and RNA electrophoresis.
- Due to a magnetic sensor, current can only flow to the electrodes while the lid is open.
- When the lid is removed or opened while the system is operating, the current is promptly cut off.
Isoelectric focusing electrophoresis system SymphonyIEF (Manufacturer: Hercuvan)
Features
- A versatile machine called SymphonyIEF Isoelectric Focusing can handle most IEF needs, from small-scale to high-throughput operations.
- It works with IEF and PAGE horizontal precast gels when the electrode frame is attached directly to the cooling plate.
References
- Viswanathan, S., Unlü, M., & Minden, J. S. (2006). Two-dimensional difference gel electrophoresis. Nature protocols, 1(3), 1351–1358. https://doi.org/10.1038/nprot.2006.234
- Büyükköroğlu, G., Dora, D. D., Özdemir, F., & Hızel, C. (2018). Techniques for Protein Analysis. Omics Technologies and Bio-Engineering, 317–351. doi:10.1016/b978-0-12-804659-3.00015-4
- https://javalab.org/en/dna-electrophoresis/
- https://conductscience.com/introduction-to-electrophoresis/
- https://study.com/learn/lesson/agarose-gel-electrophoresis-steps-purpose.html
- https://www.vedantu.com/biology/sds-page
- https://uomustansiriyah.edu.iq/media/lectures/6/6_2021_09_15!11_46_27_PM.docx
- https://sciencing.com/disadvantages-gel-electrophoresis-8003362.html
- https://www.medicalexpo.com/prod/hercuvan/product-113272-925705.html
- https://www.medicalexpo.com/prod/g-biosciences/product-301595-1013667.html
- https://www.medicalexpo.com/prod/inovialab/product-130336-1026357.html
- https://www.medicalexpo.com/prod/bioevopeak/product-301335-1060455.html

gel electrophoresis and immunoelectrophoresis me difference