- Electrophoresis through agarose or polyacrylamide gels is a standard method used to separate, identify and purify biopolymers, since both these gels are porous in nature.
- Polyacrylamide gels are chemically cross-linked gels formed by the polymerization of acrylamide with a cross-linking agent, usually N,N’-methylenebisacrylamide.
- The reaction is a free radical polymerization, usually carried out with ammonium persulfate as the initiator and N,N,N’,N’-tetramethylethylendiamine (TEMED) as the catalyst.
- Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility.
- The most commonly used form of polyacrylamide gel electrophoresis is the Sodium dodecyl suplhate Polyacrylamide gel electrophoresis (SDS- PAGE) used mostly for the separation of proteins.
Interesting Science Videos
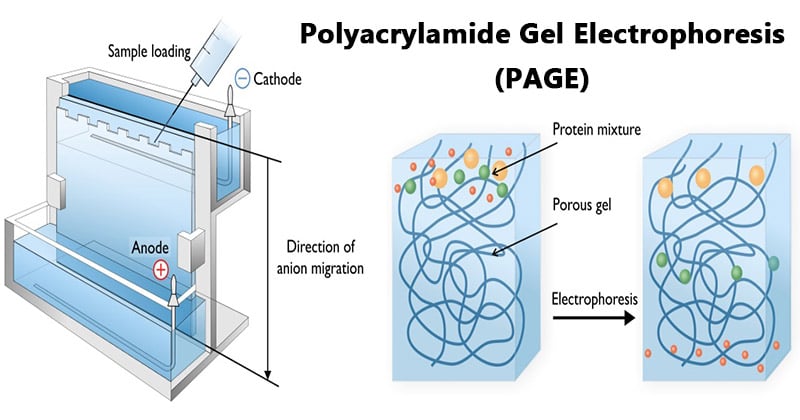
Principle of Polyacrylamide Gel Electrophoresis (PAGE)
SDS-PAGE (Polyacrylamide Gel Electrophoresis), is an analytical method used to separate components of a protein mixture based on their size.
The technique is based upon the principle that a charged molecule will migrate in an electric field towards an electrode with opposite sign. The general electrophoresis techniques cannot be used to determine the molecular weight of biological molecules because the mobility of a substance in the gel depends on both charge and size.
To overcome this, the biological samples needs to be treated so that they acquire uniform charge, then the electrophoretic mobility depends primarily on size. For this different protein molecules with different shapes and sizes, needs to be denatured (done with the aid of SDS) so that the proteins lose their secondary, tertiary or quaternary structure .The proteins being covered by SDS are negatively charged and when loaded onto a gel and placed in an electric field, it will migrate towards the anode (positively charged electrode) are separated by a molecular sieving effect based on size. After the visualization by a staining (protein-specific) technique, the size of a protein can be calculated by comparing its migration distance with that of a known molecular weight ladder (marker).
Requirements for Polyacrylamide Gel Electrophoresis (PAGE)
- Acrylamide solutions (for resolving & stacking gels).
- Isopropanol / distilled water.
- Gel loading buffer.
- Running buffer.
- Staining, destaining solutions.
- Protein samples
- Molecular weight markers.
The equipment and supplies necessary for conducting SDS-PAGE includes:
- An electrophoresis chamber and power supply.
- Glass plates (a short and a top plate).
- Casting frame
- Casting stand
- Combs
Steps Involved in Polyacrylamide Gel Electrophoresis (PAGE)
- Sample preparation
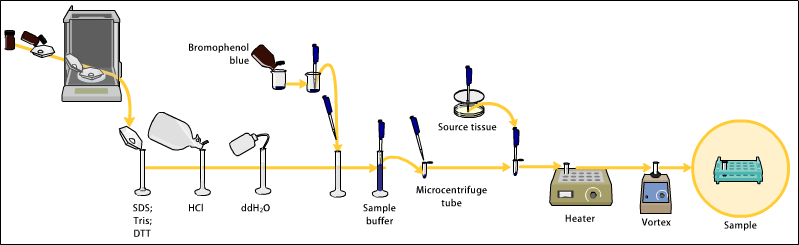
- Samples may be any material containing proteins or nucleic acids.
- The sample to analyze is optionally mixed with a chemical denaturant if so desired, usually SDS for proteins or urea for nucleic acids.
- SDS is an anionic detergent that denatures secondary and non–disulfide–linked tertiary structures, and additionally applies a negative charge to each protein in proportion to its mass. Urea breaks the hydrogen bonds between the base pairs of the nucleic acid, causing the constituent strands to anneal. Heating the samples to at least 60 °C further promotes denaturation.
- A tracking dye may be added to the solution. This typically has a higher electrophoretic mobility than the analytes to allow the experimenter to track the progress of the solution through the gel during the electrophoretic run.
- Preparation of polyacrylamide gel
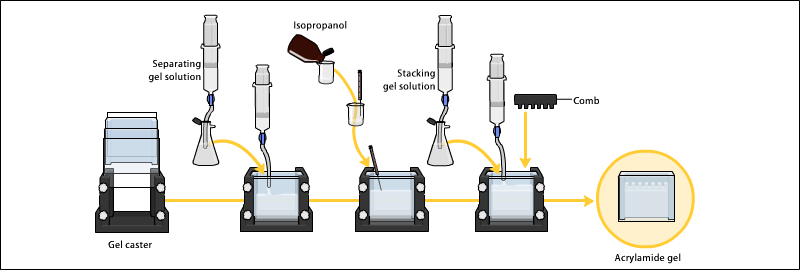
- The gels typically consist of acrylamide, bisacrylamide, the optional denaturant (SDS or urea), and a buffer with an adjusted pH.
- The ratio of bisacrylamide to acrylamide can be varied for special purposes, but is generally about 1 part in 35. The acrylamide concentration of the gel can also be varied, generally in the range from 5% to 25%.
- Lower percentage gels are better for resolving very high molecular weight molecules, while much higher percentages of acrylamide are needed to resolve smaller proteins,
- Gels are usually polymerized between two glass plates in a gel caster, with a comb inserted at the top to create the sample wells.
- After the gel is polymerized the comb can be removed and the gel is ready for electrophoresis.
- Electrophoresis
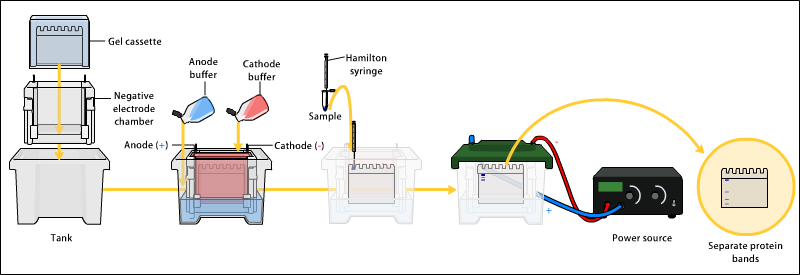
- Various buffer systems are used in PAGE depending on the nature of the sample and the experimental objective.
- The buffers used at the anode and cathode may be the same or different.
- An electric field is applied across the gel, causing the negatively charged proteins or nucleic acids to migrate across the gel away from the negative and towards the positive electrode (the anode).
- Depending on their size, each biomolecule moves differently through the gel matrix: small molecules more easily fit through the pores in the gel, while larger ones have more difficulty.
- The gel is run usually for a few hours, though this depends on the voltage applied across the gel.
- After the set amount of time, the biomolecules will have migrated different distances based on their size.
- Smaller biomolecules travel farther down the gel, while larger ones remain closer to the point of origin.
- Biomolecules may therefore be separated roughly according to size, which depends mainly on molecular weight under denaturing conditions, but also depends on higher-order conformation under native conditions.
- Detection
- Following electrophoresis, the gel may be stained (for proteins, most commonly with Coomassie Brilliant Blue or autoradiography; for nucleic acids, ethidium bromide; or for either, silver stain), allowing visualization of the separated proteins, or processed further (e.g. Western blot).
- After staining, different species biomolecules appear as distinct bands within the gel.
- It is common to run molecular weight size marker sof known molecular weight in a separate lane in the gel to calibrate the gel and determine the approximate molecular mass of unknown biomolecules by comparing the distance traveled relative to the marker.
Applications of Polyacrylamide Gel Electrophoresis (PAGE)
- Measuring molecular weight.
- Peptide mapping.
- Estimation of protein size.
- Determination of protein subunits or aggregation structures.
- Estimation of protein purity.
- Protein quantitation.
- Monitoring protein integrity.
- Comparison of the polypeptide composition of different samples.
- Analysis of the number and size of polypeptide subunits.
- Post-electrophoresis applications, such as Western blotting.
- Staining of Proteins in Gels with Coomassie G-250 without Organic Solvent and Acetic Acid.
- Pouring and Running a Protein Gel by reusing Commercial Cassettes.
- Selective Labelling of Cell-surface Proteins using CyDye DIGE Fluor Minimal Dyes.
- Detection of Protein Ubiquitination.
Advantages of Polyacrylamide Gel Electrophoresis (PAGE)
- Stable chemically cross-linked gel
- Greater resolving power (Sharp bands)
- Can accommodate larger quantities of DNA without significant loss in resolution
- The DNA recovered from polyacrylamide gels is extremely pure
- The pore size of the polyacrylamide gels can be altered in an easy and controllable fashion by changing the concentrations of the two monomers.
- Good for separation of low molecular weight fragments
Disadvantages of Polyacrylamide Gel Electrophoresis (PAGE)
- Generally more difficult to prepare and handle, involving a longer time for preparation than agarose gels.
- Toxic monomers
- Gels are tedious to prepare and often leak
- Need new gel for each experiment Stable chemically cross-linked gel
References
- http://elte.prompt.hu/sites/default/files/tananyagok/IntroductionToPracticalBiochemistry/ch07s03.html
- https://www.wou.edu/las/physci/ch462/Gel%20Electrophoresis.pdf
- https://www.slideshare.net/mbn1994/introduction-principle-instrumentation-and-applications-of-sdspage-55728195
- https://en.wikipedia.org/wiki/Polyacrylamide_gel_electrophoresis https://msu.edu/course/css/451/Lecture/PT-electrophoresis%20(2009).pdf
- http://library.umac.mo/ebooks/b28050459.pdf
- http://vlab.amrita.edu/?sub=3&brch=186&sim=319&cnt=1

Good work, very helpful!
Excellent 👍👌👌
Loved the content Bro
Very useful notes.. Simply clearly and understand paragraph
Nice notes