Fluorimetry is a scientific and analytical technique used to detect and measure the fluorescent light emitted by the sample (lying in the visible spectrum) with the aid of ultraviolet rays.
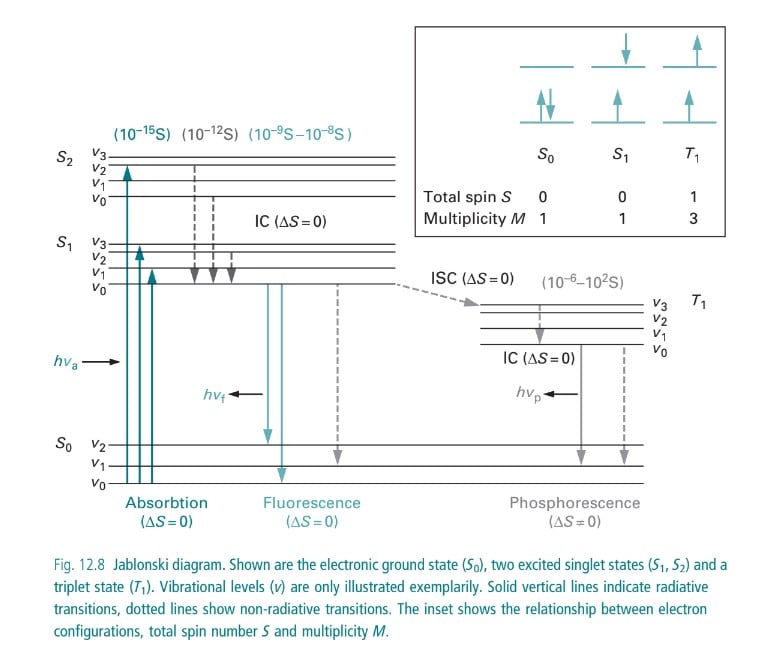
Here, the wavelength of the emitted light by the substance is greater than the absorbed light. This phenomenon is called luminescence (cold light). In luminescence, light is produced at low temperatures hence the light produced is called “cold light” or “light without heat”.
With the measurement of the intensity along with the wavelength of the emitted fluorescence, the properties and composition of the sample can be studied.
Interesting Science Videos
Luminescence Types
Luminescence is broadly categorized into types:
- Fluorescence
- A beam of light incident on materials emits visible radiations, the phenomenon called fluorescence, and the substance exhibiting phenomenon are called fluorescent substances.
- It is instantaneous.
- It stars soon after the absorption of light and stops as soon as the incident light is cut off.
- Phosphorescence
- It is delayed fluorescence.
- The light continues to be emitted from the substance, even after the light is cut off.
- Such substances are called phosphorescent substances.
Fluorimetry Principle
Fluorimetry is based on the principle of emission of light by a substance after the absorption of light of a specific wavelength.
With the absorption of light, the fluorophores in the samples get excited moving to a state of higher energy.
The excited state being unstable the electron relaxes to its ground state with lower energy releasing energy in the form of photons.
Since the energy is lost during this process, light with lower energy but longer wavelengths is emitted by the fluorochrome in comparison to the absorbed light while returning to the ground state.
The light emitted is called fluorescence and the detecting instrument is known as fluorimeter.
When the light of a suitable frequency falls, it is absorbed by the sample. This leads to the excitation of electrons from the ground state to the excited electronic state.
The following three phenomena can occur to the excited electron depending upon the molecule excited:
Deactivation without any emission of radiation: Excited state being relatively unstable the molecules return to the ground state through collisional deactivation without the emission of radiation.
Fluorescence: Excited molecules while returning to the ground state emit visible light photons.
Phosphorescence: Molecule in an excited state (relatively stable excited state) goes under transition and returns to ground state after some time emitting visible radiation.
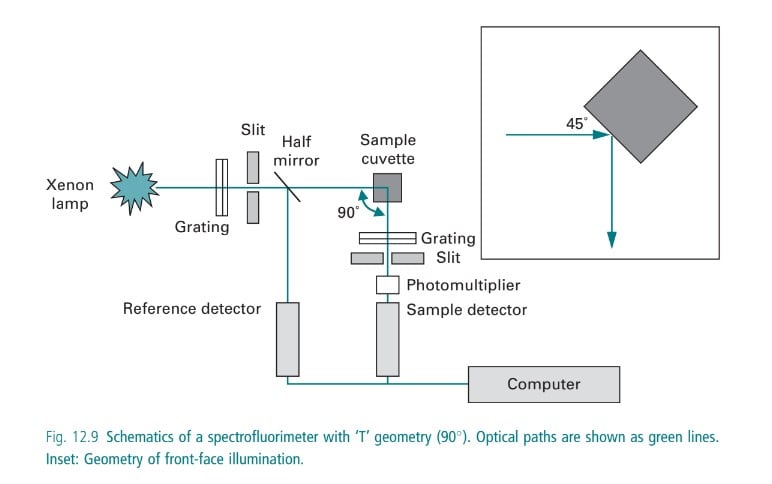
In case the sample emits radiation, a fluorimeter is used to detect the fluorescence in the sample. A fluorometer consists of a mercury vapor lamp, condensing lens, primary and secondary filter, sample container, and a receiving photocell. The primary filter selects the UV rays, whereas the secondary filter transmits visible radiation absorbing the incident ultraviolet radiation. The UV radiation emitted from a mercury vapor lamp passes from the primary filter through a sample container from which fluorescent radiation is obtained. The emitted fluorescent radiation is passed through a secondary filter that absorbs the primary radiant energy (i.e. UV rays from the source) but transmits the fluorescent radiation which is received by a photocell placed in a position right angle to the incident beam. A sensitive galvanometer is employed for the measurement of the output of the photocell.
Two types of spectrums are observed in fluorescence spectroscopy:
- Excitation or absorption spectrum
- Emission spectrum
Fluorophores and their role in fluorescence
Fluorophores: It is a chemical compound that re-emits light of lower energy after excitation by light. They are chemically diverse.
Fluorophores are categorized into three categories.
- Organic dyes: It includes fluorescein, rhodamine, and AMCA (Amine reactive dyes). Also, the derivatives of these organic compounds are used for improved solubility and photostability.
- Biological fluorophores: Fluorophores found in living organisms. It is a natural component of a cell be it a protein or a pigment. E.g.: Green fluorescent protein from Jellyfish.
- Quantum dots: They are nanocrystals. It is more photostable and can remain fluorescent for a longer period.
Jablonski diagram and energy levels in fluorescence
Instrumentation in Fluorimetry
Source of Light
Hydrogen lamps and deuterium lamps
A glass tube containing hydrogen or deuterium gas consists of a pair of electrodes. With the passes of current in electrodes, discharge of electrons occurs exciting the gas molecules in the glass tube hence resulting in the emission of radiation which lies on the visible range and can be observed through our eyes.
Wavelength: 160-800 nm
A quartz window must be employed.
GNR lamp
Quartz or fused silica tubes consist of two tungsten electrodes and a xenon gas stored under pressure which emits radiation with a higher intensity (500nm) as compared to a hydrogen discharge lamp.
Wavelength: 750-1000 nm.
Tungsten lamp (Halo lamp)
It is a filament enclosed in a quartz vessel with inert gas and a trace amount of halogen mainly iodine or bromine. It is an incandescent source of light.
Mercury vapour lamp
They are the ideal source that provides high-intensity light in the visible range. It consists of two tungsten electrodes placed in the mercury vapor and pure argon gas medium enclosed in a silica glass tube mostly elliptical in shape. It provides white light clear and of high intensity. It is used in filter fluorimeters.
Xenon arc lamp
It is the source of more intense radiation in comparison to mercury vapor lamps used in spectrofluorometers.
Tungsten lamp
It is used in low-cost instruments where the excitation has to be done in a fluorimeter.
Filter and Monochromator
Filter
It allows the selection of light of a specific wavelength to pass through it by absorbing the unwanted light. Primary and secondary filters are used to obtain the light of a specific wavelength for a particular analysis.
Primary filter: absorbs visible radiation and transmits UV radiation.
Secondary filter: absorbs UV radiation and transmits visible radiation.
Monochromator
A monochromator isolates a specific range of wavelengths of radiation and usually is used in converting polychromatic light into monochromatic light.
Excitation monochromators: Provides suitable radiation for the excitation of molecules.
Emission monochromators: Isolate only the radiation emitted by the fluorescent molecules.
Cuvettes or sample handlers
They are rectangular or cylindrical structures with two rough and two smooth sides and are used in the handling of the samples. These are usually made up of fused silica, glass, or quartz.
Detector
This device is used in transforming light energy into electrical signals which are observed on the recorder.
Characteristics of ideal detector
- Gives quantitative response.
- High sensitivity
- Low noise
- Short response time
Commonly used detectors:
Barrier cell/ Photovoltaic cell
- Consists of a silver or thin layer of gold-coated metallic film that acts as an electrode.
- Consists of two electrodes
- Both layers are separated by the layer of selenium acting as the semiconductor.
- Falling of UV rays on the selenium layer, mobilizes the electrons which are taken up by the metallic electrodes creating a potential difference and hence resulting in the flow of electrons.
- Flow of current is detected through the galvanometer, where the current is directly proportional to the intensity and wavelength of light falling on it.
Photo tubes/ Photo emissive tube
- Consists of an evacuated glass tube with a photocathode and a collector anode.
- The photocathode surface is coated with elements such as caesium, silver oxide also its mixtures.
- On falling of radiant energy on the cathode (photosensitive), emission of electrons occurs, which are attracted to the anode causing the flow of electrons.
- More sensitive the barrier layer cell.
Photomultiplier tube
- It multiplies the photoelectrons through the secondary emission of electrons.
- Vacuum tube consists of a fixed primary photocathode which receives radiation from the sample.
- About 8-10 dynodes are fixed with an increasing potential of 75-100V higher than the preceding one.
- Near the last dynode, a fixed electron collector is present which is extremely sensitive to light.
- It detects weaker or lower radiation, even 200 times weaker than that could be done using a photovoltaic cell.
Factors affecting fluorescence
- Structure
- Nature of solvent
- Viscosity
- Temperature
- Concentration of fluorophores
- Intensity
- Quenchers
Structure
Flexibility and rigidity of the molecule influence fluorescence and phosphorescence signal.
A high degree of flexibility: It decreases fluorescence. Higher collision due to high flexibility can lower the fluorescence.
More rigid structure: It increases fluorescence. A lower probability of collisions in a rigid structure increases the fluorescence.
Nature of solvent
Polarity: The polar solvent, the lower the energy required for the activation of the electron. Hence higher polarity increases the fluorescence.
Viscosity: Highly viscous substances prevent the deactivation due to collision among particles, hence increasing the fluorescence.
Temperature
High temperature: At higher temperatures, collision deactivation occurs due to an increase in movement and velocity of molecules due to high kinetic energy. It decreases the fluorescence.
Therefore, lower temperatures are preferred for increases fluorescence.
Concentration of fluorophores
Fluorescence spectroscopy works most accurately at very low concentrations of emitting fluorophores.
The higher the concentration of fluorophores in the sample, the higher the number of excited fluorophores. A higher number of excited fluorophores increases the intensity of fluorescence emitted. However, it is valid only when the absorbance is less than 0.02.
Intensity of incident light
An increase in intensity increases the fluorescence of the sample.
Quenchers
Quenching: Reduction of fluorescence intensity due to the presence of particles other than the analyte.
Collision quenching: Reduction in fluorescence due to collisions between fluorescent substances and halides.
Static quenching: Reduction in fluorescence due to complex formation between fluorescent molecules and other molecules.
Applications of Fluorimetry
In biological sciences
Biochemical analysis: Fluorimetry helps in the analysis of concentration, structure, and interactions of compounds such as proteins, and nucleic acids, and their quantification.
Characterization: It allows the characterization and analysis of properties of materials such as nanomaterials, and semiconductors. It also aids in the monitoring of fluorescence under different conditions of pressure, temperature, and humidity.
Biological imaging: It is useful in microscopy for the visualization of structures such as protein, DNA, or any other specific molecule.
Environmental Sciences
Detection of pollutants: Fluorimeter detects the presence and concentration of chemicals, pollutants such as polycyclic aromatic hydrocarbons (PAH), heavy metals, and other contaminants in water, soil, and air. It also is used in carrying out qualitative and quantitative analyses of compounds present in air cigarette smoke, and automobile exhaust.
Medical and pharmaceutical sciences
Drug discovery: Fluorimetry allows for the screening and characterization of potential drug candidates. It also allows for the study of the binding affinity of certain compounds to target proteins, monitor the efficacy of enzymes, etc.
Vitamin identification: It is also applicable in the ascertainment of vitamins such as thiamin (B1), and riboflavin (B2) in foods such as cereals, meat, etc.
Cancer diagnosis: Laser-induced fluorescence spectroscopy can be used in the detection of tumors, helping in the early diagnosis of cancer.
Nuclear research
It is used in the determination of uranium salts in the field of nuclear research.
Organic and inorganic studies
It aids in the study of aluminum alloys, chromium, and manganese in steel, thiamine assay, investigation of the rate of reactions, Determination of uranium salts; aluminum in alloys; chromium and manganese in steel; beryllium in silicates, etc.
Fluorimetry has recently gained recognition in the investigation of synthetic polymers. It allows for the study of glass transition phenomena, polymer compatibility, and conformational mobility of flexible chains.
Advantages
- A fluorometer is useful in the quantification of fluorescent species.
- It is economic.
- It is used in rapid diagnosis.
- The decay time and concentration of the particles in the sample can be measured.
- It is a high-precision technique.
Limitation
- It is only useful for the analysis of fluorescent species.
- The life span of fluorophores is short.
References
- Fluorimetry. Instrumental Methods of Analysis. Retrieved from https://monad.edu.in/img/media/uploads/IMA(BP-701)%20(U-1P-3)Fluorimentry.pdf. Accessed on 24th January 2024.
- Fluorophores. Nano temper. Retrieved from https://nanotempertech.com/nanopedia/fluorophores/#:~:text=A%20fluorophore%20is%20a%20molecule,Fluorophores%20are%20chemically%20diverse. Accessed on 20th January 2024.
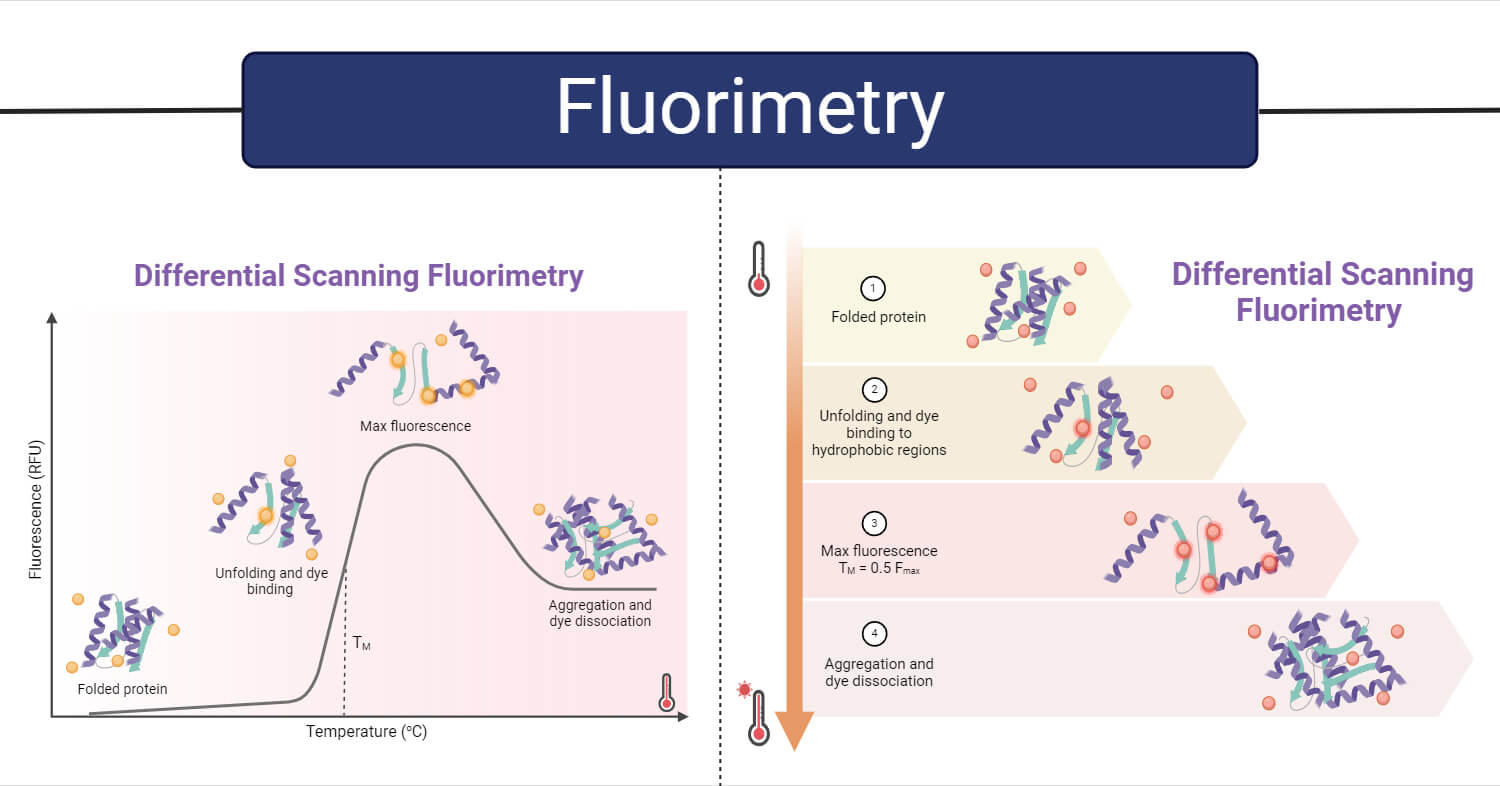
- Gao, K., Oerlemans, R., & Groves, M. R. (2020). Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophysical reviews, 12(1), 85-104. Retrieved from https://link.springer.com/article/10.1007/s12551-020-00619-2. Accessed on 29th January 2024.
- Karki, G. (2020). Fluorimetry- Principle and Applications. Retrieved from https://www.onlinebiologynotes.com/fluorimetry-principle-and-applications/. Accessed on 26th January 2024.
- Morawetz, H. (1979). Some applications of fluorimetry to synthetic polymer studies. Science, 203(4379), 405-410. DOI: 10.1126/science.203.4379.405
- What are the types of fluorophores? (2022). Retrieved from https://www.aatbio.com/resources/faq-frequently-asked-questions/what-are-the-types-of-fluorophores. Accessed on 28th January 2024.
- What is Fluorimetry? Theory and Application. (2023). Retrieved from https://pharmasiksha.com/fluorimetry-theory-application/. Accessed on 20th January 2023.
- Wilson, K. and Walker, J. (2010). Principles and Techniques of Biochemistry and Molecular Biology. 7th Edition. Cambridge University Press.
