Interesting Science Videos
What is the Esculin Hydrolysis Test?
Esculin hydrolysis is a useful test in the differentiation of both gram-positive and gram-negative bacteria covering a broad spectrum of aerobes, facultative anaerobes, and anaerobes. Esculin hydrolysis is utilized as a taxonomic tool in the identification of a wide variety of microorganisms, including the family Enterobacteriaceae, genera Streptococcus and Listeria, non-fermentative gram-negative bacilli, and anaerobes. Esculin hydrolysis is a differential test that differentiates bacteria on the basis of their ability to hydrolyze esculin.
- This test can be made selective by adding bile to the medium, which is called the bile esculin test.
- Hydrolysis of esculin by bacteria can be determined in growth-supporting media like Vaughn-Levine, bile-esculin, or Pfizer selective enterococcus media or by non-growth-supporting methods like PathoTec and rapid spot tests.
- The esculin hydrolysis test is based on the hydrolysis of esculin (a glucoside) into glucose and esculetin by a microorganism that has constitutive β-glucosidase or esculinase enzyme.
Objectives of Esculin Hydrolysis Test
- To detect the ability of an organism to hydrolyze esculin by the production of esculinase enzyme.
- To differentiate and identify members of the Enterobacteriaceae family.
Principle of Esculin Hydrolysis Test
- Esculin is a β-glucose-6,7-dihydroxycoumarin, a compound derived from the horse chestnut tree (Aesculus hippocastanum).
- The compound can be enzymatically hydrolyzed at the 8-glucose linkage to yield two products, esculetin, and glucose.
- Esculin hydrolysis is commonly determined by detecting the end product esculetin.
- Esculetin combines with ferric ions, generally incorporated in the medium as ferric ammonium citrate, to produce a brown-black colored compound.
- Alternatively, the end product glucose can be determined by detecting the change in pH resulting from its fermentation. This method is limited to those organisms that ferment glucose.
- In the esculin hydrolysis test performed in the laboratory, the reaction between esculetin and ferric ions forms a black-colored compound that can be detected on the esculin agar or via the Esculin spot test.
Microorganisms Tested
- Gram-positive cocci in chains, which are catalase-negative and morphologically identified as presumptive Streptococcus bovis.
- Microorganisms that are alpha- or gamma-hemolytic, Gram-positive cocci as part of differentiation of enterococci from other pyrrolidonyl-β-naphthylamide (PYR)-positive organism.
- Non-spore-forming, hemolytic, Gram-positive rods that are catalase-positive and morphologically presumed as Listeria.
- Positive blood cultures with Gram-positive cocci in chains or Gram-positive rods, to rapidly (4 hours) identify enterococci and Listeria.
- Esculin hydrolysis for the identification of oxidase-positive aerobic Gram-negative rods, including Aeromonas spp. and yellow-pigmented non-glucose-fermenting rods.
Media, Reagent, and Supplies Used
Media Used
- Esculin Agar is used for the detection of the hydrolysis of esculin. The medium is a differential medium and can be made selective by adding bile.
- The composition of the Esculin Agar is given below:
| S.N | Ingredients | Gram/liter |
| 1. | Casein enzymic hydrolysate | 13.0 |
| 2. | Yeast extract | 5.0 |
| 3. | Beef heart infusion (solids) | 2.0 |
| 4. | Sodium chloride | 5.0 |
| 5. | Ferric citrate | 0.5 |
| 6. | Agar | 15.0 |
| Final pH at 25°C: 7.3 ±0.2 | ||
Reagent Used
- For the esculin spot test, 0.02% esculin solution is prepared in distilled water.
Supplies Used
- Long-wave (360-nm) UV light
- Sterile sticks, needles, or inoculating loops
- Pasteur pipettes or drinking straws
- Boiling heat block
- Incubators at 35 and 30°C
Procedure of Esculin Hydrolysis Test
A. Preparation of the media
- In a beaker, 41.5 grams of the dehydrated powder or lab-prepared media is added to 1000 milliliters of distilled or deionized water.
- The mixing is followed by heating with agitation up to boiling to dissolve the medium completely.
- The solution is then dispensed into screw-capped tubes (about 3 ml each) and sterilized in an autoclave at 15 lbs pressure (121°C) for 15 minutes.
- The tubes are taken out after autoclaving and cooled at a slanted position to a temperature of about 40-45°C. The position should be maintained at an angle to achieve butts of 1.5 – 2.0 cm depth.
B. Hydrolysis Test
Esculin hydrolysis can be observed either through tube test or esculin spot test. The spot test is a rapid test.
1. Tube Test
- A light inoculum is derived from an 18-24 hour culture with a sterile inoculating needle from the center of a well-isolated colony.
- The esculin agar tubes are inoculated by streaking the surface of the slant with the light inoculum picked from the culture plate.
- The caps of the test tubes should be capped loosely to ensure adequate aeration.
- The inoculated tubes are then incubated in the air at 35-37°C for 24 hours (or up to 7 days for slow-growing Gram-negative rods and anaerobes), and the color change is observed.
- If esculin broth without iron (III) citrate is used, the tubes are observed daily for loss of fluorescence with UV light.
- In the case of loss of fluorescence, 2 or 3 drops of 1% ferric ammonium citrate are added to the tube, and the change in color is observed.
2. Esculin Spot Test
- A 0.02% esculin solution is made in distilled water which is then sterile by autoclaving or by filter-sterilization.
- A filter paper is placed on a standard microscope slide and positioned on supporting glass rods.
- Esculin solution is pipetted over the paper while avoiding the over-saturation of the paper.
- The inoculum is derived from a 24-h bacterial colony with a wooden stick that is rubbed in the center of the filter paper.
- The slide is then incubated at 37°C for about 10-15 minutes. Although Klebsiella generally yielded a positive test within 10 to 15 min, one should hold the test 30 min before calling it negative.
- Using a hand-held Wood lamp in subdued light, the spot is observed for the loss of fluorescence.
Control organisms
- Positive: Enterococcus faecalis
- Negative: Escherichia coli
Result Interpretation of Esculin Hydrolysis Test
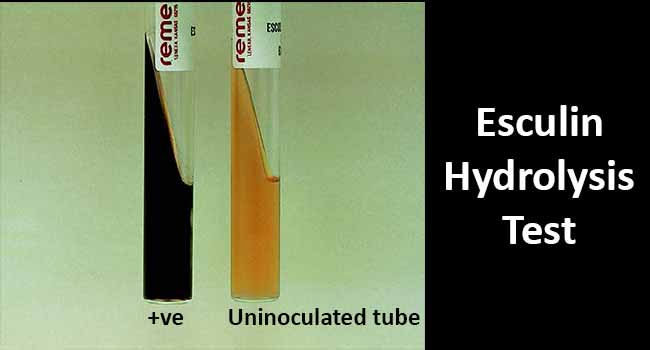
Figure: Result Interpretation of Esculin Hydrolysis Test. Image Source: Bailey and Scott’s Diagnostic Microbiology. Elsevier.
- The blackening of the medium demonstrates a positive tube test in the esculin medium with ferric ammonium citrate.
- The lack of color change demonstrates a negative tube test.
- A positive spot test is demonstrated by the loss of fluorescence, resulting in a black colored spot under the UV light.
- A negative test is demonstrated by a bright fluorescence, indicating no decrease in the esculin concentration.
Uses of Esculin Hydrolysis Test
- Esculin Hydrolysis test is used in the identification of a wide variety of microorganisms, including the family Enterobacteriaceae, genera Streptococcus and Listeria, non-fermentative gram-negative bacilli, and anaerobes.
- The test can be performed to determine an organism’s ability to hydrolyze esculin or to produce the esculinase enzyme.
- The test can be made selective for Streptococcus species by the addition of the bile solution.
Limitations of Esculin Hydrolysis Test
- Several organisms produce H2S during metabolism, which might react with iron to produce a black complex and interfere with the interpretation of the esculin hydrolysis test. Therefore, for Gram-negative rods, check tubes showing darkening after the addition of the reagent under UV light.
- Some microorganisms, such as E. coli, have an inducible β-glucosidase and will give a positive result only after prolonged incubation (up to 7 days). However, prolonged incubation should not be used if the test is being used to detect only constitutive β-glucosidase.
References and Sources
- Esculin agar. M1386. HiMedia Laboratories.
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3.DOI:10.1128/9781555818814.ch3.17.1
- Edberg, S. C., Gam, K., Bottenbley, C. J., & Singer, J. M. (1976). Rapid spot test for the determination of esculin hydrolysis. Journal of clinical microbiology, 4(2), 180–184.
- S C Edberg, K Gam, C J Bottenbley, J M Singer. Rapid spot test for the determination of esculin hydrolysis. Journal of Clinical Microbiology. Aug 1976, 4 (2) 180-184.
- 8% – https://microbenotes.com/bile-esculin-test/
- 2% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC274422/
- 1% – https://quizlet.com/225068222/biochemical-tests-flash-cards/
- 1% – https://jcm.asm.org/content/jcm/25/6/1107.full.pdf
- 1% – https://en.wikipedia.org/wiki/Viridans_streptococci
- 1% – http://www.austincc.edu/microbugz/bile_esculin_test.php
- <1% – https://www.researchgate.net/publication/236587726_Development_of_rapid_phenotypic_system_for_the_identification_of_Gram-negative_oxidase-positive_bacilli_in_resource-limited_settings
- <1% – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC274715/
- <1% – https://tuttnauer.com/blog/autoclave
- <1% – https://study.com/academy/lesson/gram-negative-bacilli-characteristics-types-examples.html
- <1% – https://quizlet.com/285520382/lab-final-review-flash-cards/
- <1% – https://microbenotes.com/
- <1% – https://link.springer.com/article/10.1007/BF02350787
- <1% – https://jcm.asm.org/content/jcm/6/2/111.full.pdf
- <1% – https://en.wikipedia.org/wiki/Agar_plate
- <1% – https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/EsculinAgar.htm
