Interesting Science Videos
What is the Bile Esculin Test?
The bile-esculin test is a biochemical test performed to differentiate Enterococci and group D Streptococci from non-group D viridans group Streptococci on the basis of their ability to hydrolyze esculin.
- Many organisms are capable of hydrolyzing esculin, but only a few of them can do so in the presence of bile (4% bile salts or 40% bile.). Thus, this property is utilized to identify organisms of a particular group.
- The Bile-esculin test is performed on a selective differential agar; bile esculin agar, which consists of bile as well as esculin.
- The agar contains different bile salts that inhibit the growth of other Gram-positive organisms and allows the selective isolation of Enterococci and Group D Streptococci.
- Esculin is a glycosidic coumarin derivative (6-beta-glucoside-7-hydroxy-coumarin) which is a fluorescent compound, and its hydrolysis can also be observed by the loss of the fluorescence.
- The Bile-esculin test has been modified through the years and has been made more rapid. Nowadays, bile-esculin disks are available that are commonly used for the rapid differentiation between Group D Streptococci and non-Group D Streptococci.
Objectives of Bile Esculin Test
- To identify Enterococci and Group D Streptococci on the basis of their ability to hydrolyze esculin in the presence of bile.
- To differentiate members of Enterococci and Group D Streptococci from other viridans or non-Group D Streptococci.
Principle of Bile Esculin Test
- The basis of the esculin test is the hydrolysis of esculin in the presence of bile salt as a result of the enzymatic action of esculinase.
- Esculin is a glucoside consisting of glucose and hydroxycoumarin linked together by an ester bond through oxygen.
- The bile esculin test selects organisms first on the basis of their ability to grow in a medium with 4% bile salts followed by the selection based on their ability to hydrolyze esculin.
- The hydrolysis of esculin results in glucose and a compound called esculetin.
- After the degradation of esculin, the esculetin produced by the hydrolysis of esculin reacts with iron ions (from ferric citrate) in the medium to form a phenolic iron complex, resulting in a dark brown or black color.
- Alternatively, esculin is a fluorescent compound, and its hydrolysis can be observed by a loss of fluorescence.
- If bile is added to the medium, the microorganism must be able to grow in its presence in order to hydrolyze esculin. The bile inhibits the growth of other Gram-positive organisms and makes the medium more selective.
- The 40% bile (equivalent to 4% oxgall) in the bile esculin medium inhibits most strains of Streptococci, other than Streptococcus bovis, but does not inhibit Enterococci or Listeria.
Microorganism tested
- Gram-positive cocci in chains, which are catalase-negative and morphologically identified as presumptive S. bovis.
- Isolates of alpha- or gamma-hemolytic, Gram-positive cocci as part of differentiation of enterococci from other pyrrolidonyl-β-naphthylamide (PYR)-positive organisms
- Non-spore-forming, hemolytic, Gram-positive rods that are catalase-positive and morphologically identified as presumptive Listeria
- Positive blood cultures with Gram-positive cocci in chains or Gram-positive rods, to rapidly (within 4 hours) identify enterococci and Listeria
- Esculin without bile for the identification of oxidase-positive aerobic Gramnegative rods, including Aeromonas and yellow-pigmented non-glucose-fermenting rods
Media, Reagents, and Supplies Used
Media Used
- Bile-esculin agar slants with iron(III) citrate. Agar plate media, such as Enterococcosel agar, have a similar formulation.
- Bile-esculin-azide agar or broth with iron(III) citrate and azide. Azide will inhibit most Gram-negative bacteria.
- Peptose-yeast-esculin broth (usually in the anaerobic atmosphere).
- Esculin agar (0.1% esculin in heart infusion basal medium) without bile or azide but with iron(III) citrate.
- The composition of Bile Esculin Agar is given below:
| S.N. | Ingredients | Gram/liter |
| 1. | Peptic digest of animal tissue | 5.0 |
| 2. | Beef extract | 3.0 |
| 3. | Esculin | 1.0 |
| 4. | Bile Salts | 40.0 |
| 5. | Ferric citrate | 0.5 |
| 6. | Bacteriological agar | 15.0 |
| Final pH at 25°C: 6.6 ±0.2 | ||
| Store at 2°C to 8°C. | ||
Reagents and Supplies Used
- Long-wave (360-nm) UV light
- 1% ferric [iron(III)] ammonium citrate if iron(III) is not incorporated into the medium
The procedure of Bile Esculin Test
Preparation of media
- In a beaker, 64.5 grams of the dehydrated powder or lab-prepared media is added to 1000 milliliters of deionized or distilled water.
- The medium is then heated up to boiling to dissolve the powder completely.
- The dissolved medium is then distributed into tubes and sterilized in an autoclave at 15 lbs pressure (121°C) for 15 minutes.
- Once the autoclaving process is complete, the tubes are taken out and cooled at a slanted position to a temperature of about 40-45°C. The position should be maintained in order to obtain butts of 1.5 – 2.0 cm depth.
Esculin Hydrolysis
- Esculin hydrolysis can be observed either through a tube test or a disk test. A disk test is a rapid test.
Tube test
- A well-isolated colony is taken from an 18-24 hour culture with a sterile inoculating needle.
- The bile esculin agar tubes are inoculated by streaking the surface of the slant with either the light inoculum picked from the culture plate.
- For enterococcus and S. bovis identification, 40% bile is used, and the tubes are inoculated with a 10-µl calibrated loopful of a 0.5 McFarland standard suspension prepared in sterile water.
- The cap of the test tubes should be left loosened to ensure adequate aeration.
- The tubes are then incubated aerobically at 35-37°C for 24 hours (or up to 7 days for slow-growing Gram-negative rods and anaerobes), and the color change is observed.
- For esculin broth without iron (III) citrate, the tubes are observed daily for loss of fluorescence.
- In the absence of fluorescence, 2 or 3 drops of 1.0% ferric ammonium citrate are added to the esculin tube, and the color change is observed.
Disk test
- The esculin disk is moistened with a single drop of distilled or deionized water. The disk, however, should not be saturated.
- Using a sterile loop, two or three well-isolated colonies are picked from an overnight (18- to 24-h) culture.
- The disk is observed for the development of a dark brown or black color after about 10 minutes at room temperature.
Result Interpretation of Bile Esculin Test
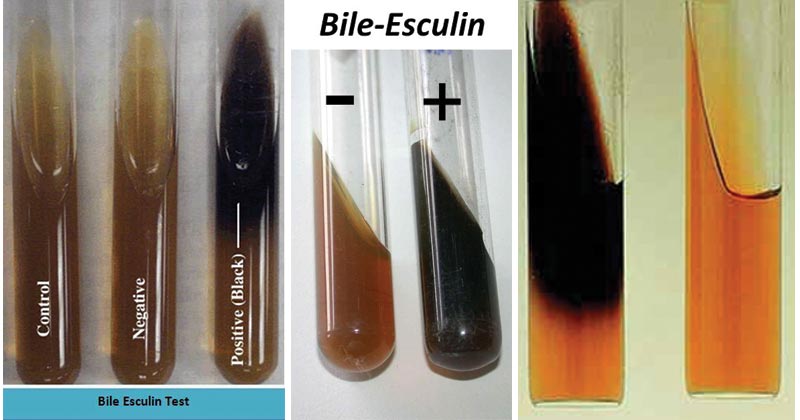
- A positive tube test in medium containing ferric ammonium citrate is indicated by the blackening of the medium.
- A negative tube test is indicated by a lack of color change. The medium will fluoresce under UV light (366 nm).
- For esculin broth without iron (III) citrate, a positive test is demonstrated either by blackening of the broth after addition of the ferric [iron(III)] reagent or by the loss of fluorescence of the medium.
- A negative test result also occurs in the bile-esculin medium if the organism cannot grow in the presence of bile, regardless of the ability to hydrolyze esculin.
- A positive disk test is indicated by the development of a dark brown or black color.
- A negative disk test remains colorless.
The following table demonstrates the growth of some bacteria and their bile esculin hydrolysis test :
| S.N. | Organism | Growth | Bile esculin hydrolysis |
| 1. | Enterococcus faecalis | Good | Positive reaction; Blackening of medium |
| 2. | Escherichia coli | Good | Negative reaction |
| 3. | Enterococcus faecium | Luxuriant | Positive reaction; Blackening of the medium around the growth. |
| 4. | Yersinia enterocolitica | Good-luxuriant | Positive reaction; Blackening of the medium. |
Uses of Bile Esculin Test
- Bile esculin test is performed as a biochemical test for the isolation of Enterococci and Group D Streptococci.
- It can also be used to differentiate these organisms from viridans Streptococci and other Gram-positive microorganisms.
- Bile Esculin Agar is a selective differential medium for the growth of organisms like Enterococcia, Listeria, and Yersinia enterocolitica.
Limitations of Bile Esculin Test
- If a large inoculum is used or if the concentration of bile is less than 40%, viridans group streptococci other than S. bovis might give a positive reaction on bile-esculin agar.
- Esculin tests without bile cannot be used to separate S. bovis (previously referred to as group D streptococci) from other viridans group streptococci.
- Several organisms might produce H2S during metabolism that might react with iron and produce a black complex, which interferes with the results of the esculin hydrolysis test and might give a false-positive result.
- Some microorganisms, such as E. coli that have β-glucosidase and will give a positive result in this test only after prolonged incubation. However, prolonged incubation should not be used if the test is being used to detect β-glucosidase in other organisms.
References and Sources
- Bile esculin agar. M1225. HiMedia Laboratories.
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3.DOI:10.1128/9781555818814.ch3.17.1
- C. Chuard, L. B. Reller. Bile-Esculin Test for Presumptive Identification of Enterococci and Streptococci: Effects of Bile Concentration, Inoculation Technique, and Incubation Time. Journal of Clinical Microbiology Apr 1998, 36 (4) 1135-1136; DOI: 10.1128/JCM.36.4.1135-1136.1998.
- 3% – https://microbeonline.com/bile-esculin-test-enterococcus-species-principle-procedure-results/
- 1% – https://www.sciencedirect.com/topics/medicine-and-dentistry/streptococcus-group-d
- 1% – https://www.coursehero.com/file/p610isqs/Alternatively-esculin-is-a-fluo-rescent-compound-and-its-hydrolysis-can-be/
- 1% – https://www.bd.com/resource.aspx?IDX=8992
- 1% – https://quizlet.com/125926606/microlab-biochemical-tests-flash-cards/
- 1% – https://microbenotes.com/nutrient-agar-principle-composition-preparation-and-uses/
- 1% – https://microbenotes.com/bile-esculin-agar/
- 1% – https://jcm.asm.org/content/36/4/1135
- 1% – https://clinicalgate.com/gram-positive-cocci/
- 1% – http://www.austincc.edu/microbugz/bile_esculin_test.php
- 1% – http://microsc.net/materials/4net/laboratory_syllabus/use_of_selective_and_differential_media.pdf
- <1% – https://vlab.amrita.edu/?sub=3&brch=73&sim=703&cnt=2
- <1% – https://pubchem.ncbi.nlm.nih.gov/compound/fluorescein
- <1% – https://persianlab.com/strepiococcus-enterococcus-and-other-catalase-negative-gram-positive-cocci/
- <1% – https://en.wikipedia.org/wiki/Bile_esculin_agar
- <1% – https://en.wikipedia.org/wiki/Agar_plate
- <1% – https://biologicalindicators.mesalabs.com/wp-content/uploads/sites/31/2014/02/Unique-Cycles-Sterilizing-Liquid-Loads.pdf
- <1% – https://asm.org/Articles/2020/January/Microbiology-Laboratory-Tips-and-Tricks-An-Organis
- <1% – https://aem.asm.org/content/aem/20/2/245.full.pdf

Have you ever heard of looking for flouescence using a UV light in BE slants?