Brilliant Green Agar medium is recommended as a primary plating medium for isolation of Salmonella species. It was first described as a selective isolation medium for Salmonella species by Kristensen et al. Kauffmann modified their formula to give a highly selective plating medium for the isolation and identification of salmonellae from feces and other pathological material, and from food and dairy products. Brilliant Green Agar should be used in parallel with other selective plating media such as Deoxycholate Citrate Agar, Hektoen Enteric Agar, XLD Agar. Bismuth Sulphite Agar is specifically recommended for Salmonella Typhi.
Interesting Science Videos
Composition of Brilliant Green Agar
| Ingredients | Gms/liter |
| Peptone | 5.000 |
| Tryptone | 5.000 |
| Yeast extract | 3.000 |
| Lactose | 10.000 |
| Sucrose | 10.000 |
| Sodium chloride | 5.000 |
| Phenol red | 0.080 |
| Brilliant green | 0.0125 |
| Agar | 20.000 |
Final pH (at 25°C): 6.9±0.2
Principle of Brilliant Green Agar
- Combination of peptone, tryptone and yeast extract makes the medium highly nutritious and supplies amino acids and long chains of peptides.
- Sodium chloride maintains the osmotic equilibrium.
- Lactose and sucrose are the fermentable carbohydrate sources.
- Phenol red serves as an acid-base indicator giving a yellow color to lactose and or sucrose fermenting bacteria.
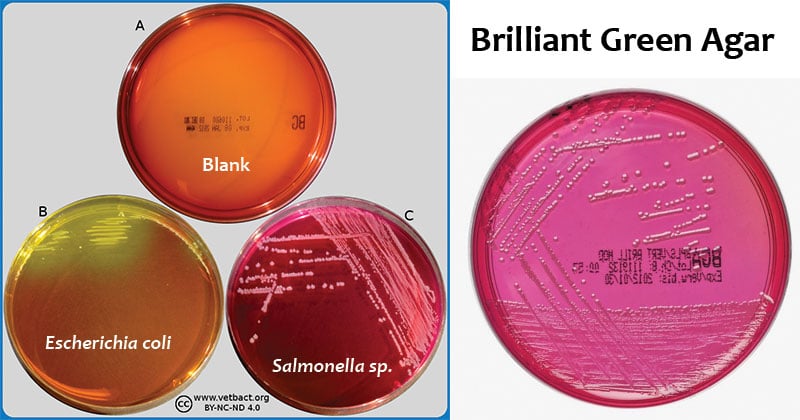
- Non-lactose fermenting bacteria develop white to pinkish red colonies within 18-24 hours of incubation.
- This medium also contains brilliant green, which inhibits the growth of the majority of Gram-negative and Gram-positive, bacteria.
- Salmonella Typhi, Shigella species, Escherichia coli, Proteus species, Pseudomonas species, Staphylococcus aureus are mostly inhibited.
Non-lactose/sucrose-fermenting organisms
Red-pink-white opaque colored colonies surrounded by brilliant red zones in the agar – most probably salmonella (but not Salmonella typhi).
Proteus and Pseudomonas species
These may grow as small red colonies.
Lactose/sucrose-fermenting organisms (normally inhibited)
Yellow to greenish-yellow colored colonies surrounded by intense yellow-green zones in the agar – Escherichia coli or Klebsiella/Enterobacter group.
Preparation and Method of Use of Brilliant Green Agar
- Suspend 58.09 grams in 1000 ml purified /distilled water.
- Heat to boiling to dissolve the medium completely.
- Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. AVOID OVERHEATING.
- Cool to 45-50°C.
- Mix well and pour into sterile Petri plates.
Examination of feces, or similar material, for salmonellae:
- Heavily inoculate a Brilliant Green Agar plate. At the same time, inoculate other plating media and tubes of Selenite Broth and Tetrathionate Broth.
- Incubate the Brilliant Green Agar plate for 18-24 hours at 35°C.
- Examine the plates and identify suspect colonies using differential tests.
- If no non-lactose fermenters are observed on the primary plate cultures, inoculate Brilliant Green Agar and other media with the enrichment cultures – then proceed as in point 3.
Examination of foods
- Pre-enrich four 25g aliquots of food in 75ml of Buffered Peptone Water and incubate at 35°C for 4-6 hours.
- Add to each sample 75ml of double-strength Selenite Cystine Broth and incubate at 43°C for 24 hours.
- Subculture to plates of Brilliant Green Agar and Bismuth Sulphite Agar.
- Incubate the plates at 35°C and examine the Brilliant Green Agar after 24 hours and the Bismuth Sulphite Agar after 48 hours.
- Look for colonies with salmonella characteristics and confirm their identity with biochemical and serological tests.
Result Interpretation on Brilliant Green Agar
| Organisms | Growth |
| Salmonella typhimurium | Good growth; red-colored colonies |
| Salmonella Abony | Good growth; pinkish white |
| Salmonella Enteritidis | Good growth; pinkish white |
| Salmonella Typhi | Poor-good; reddish pink |
| Staphylococcus aureus | Inhibited |
| Escherichia coli | Inhibited or no growth; yellowish-green |
| Enterococcus faecalis | No growth to light growth; yellowish colonies with or without yellow halos |
Uses of Brilliant Green Agar
- Brilliant Green Agar is a selective and differential medium for the isolation of Salmonella species other than S. Typhi and S. Paratyphi from clinical specimens.
- It is also recommended by APHA FDA and is in accordance with United States Pharmacopoeia. This medium is employed in testing clinical specimens.
- Heavy inocula and heavily contaminated samples can be analyzed due to the outstanding selectivity of this medium.
- It is used in the microbial limits test and with novobiocin for testing food and pharmaceutical products.
Limitations
- Being highly selective, it is recommended that this medium should be used along with a less inhibitory medium to increase the chances of recovery. Often cultures enriched in Selenite or Tetrathionate Broth are plated on Brilliant Green Agar along with Bismuth Sulphite Agar, SS Agar, MacConkey Agar.
- Salmonella typhi and Shigella species may not grow on this medium. Alternative media must be used.
- Proteus, Citrobacter and Pseudomonas species may mimic enteric pathogens by producing small red colonies.
- Biochemical and/or serological tests should be performed on isolated colonies in order to complete identification.
References
- http://himedialabs.com/TD/MU016.pdf
- http://www.oxoid.com/UK/blue/prod_detail/prod_detail.asp?pr=CM0263&org=124&c=UK&lang=EN
- https://www.bd.com/resource.aspx?IDX=8964
- http://www.dalynn.com/dyn/ck_assets/files/tech/PB84.pdf
- http://bvascientific.com/Customer/miscne/specpages/228530.pdf

what is the principle of using brilliant green togather with macconkey broth
Sir,i need pdf of all topics